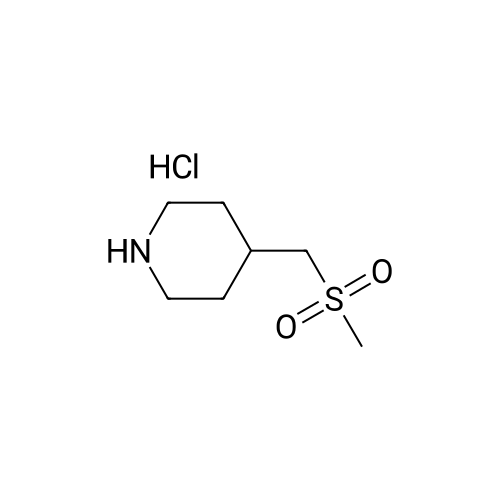
|
With potassium carbonate; In DMF (N,N-dimethyl-formamide); at 95℃; for 2.5h; |
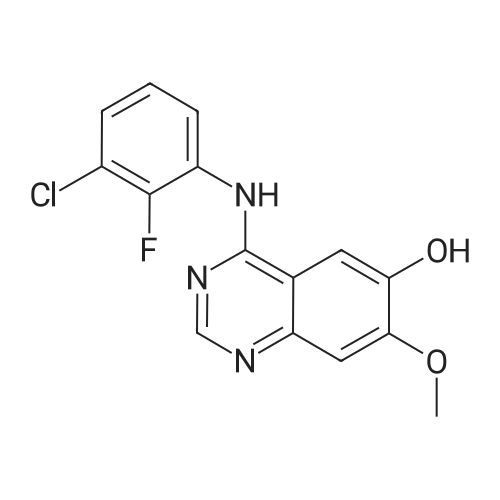
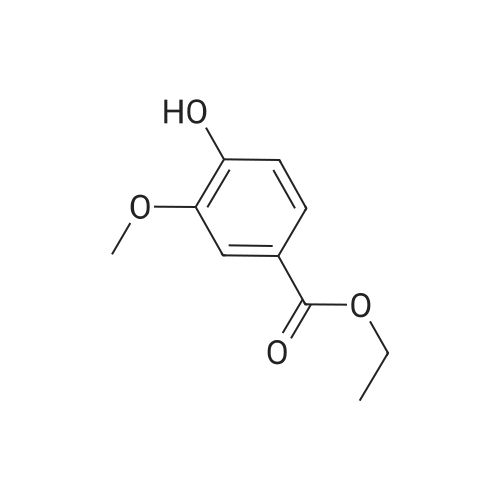
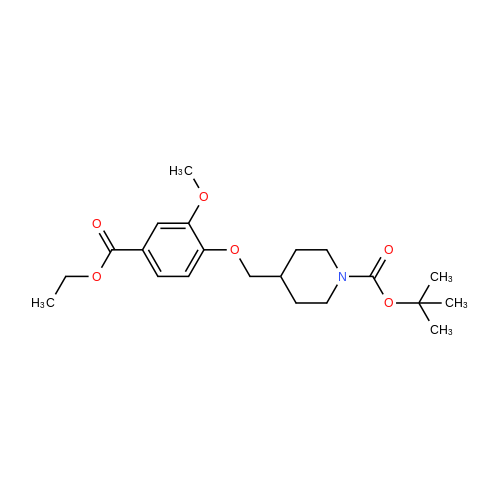
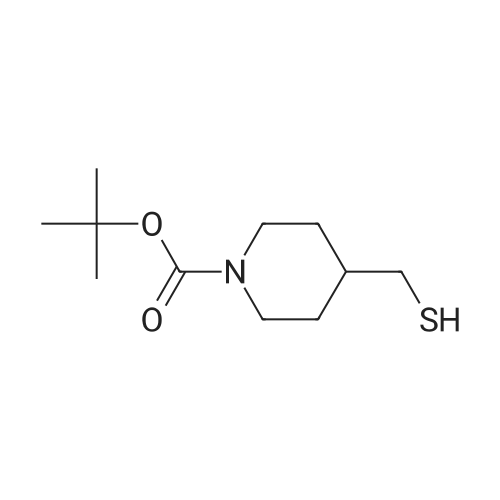
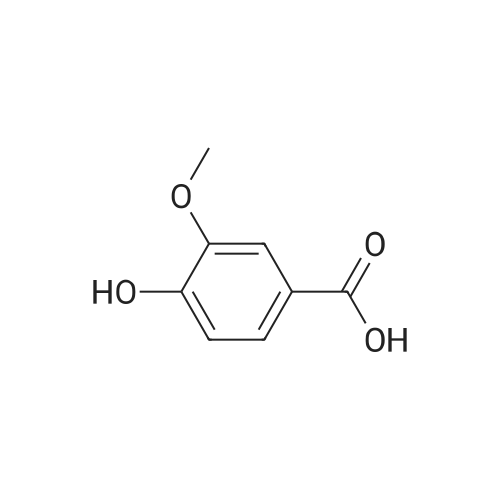
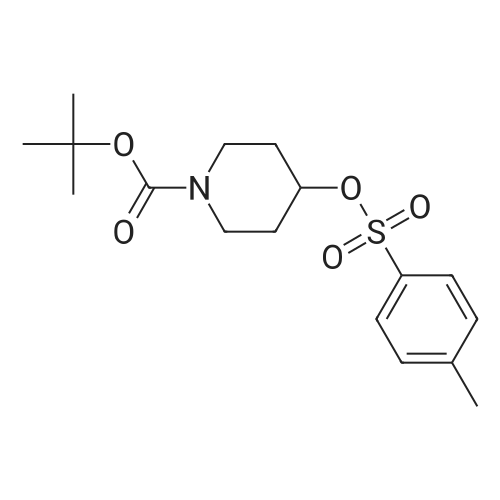
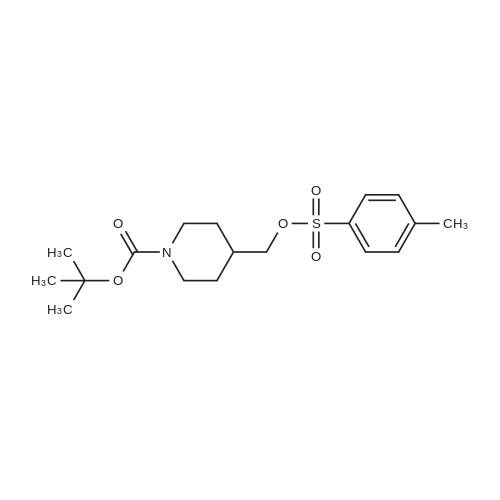
The 4-amino-6-methoxy-7-(N-methylpiperidin-4-ylmethoxy)quinazoline used as a starting material was prepared as follows: A solution of di-tert-butyl dicarbonate (41.7 g) in ethyl acetate (75 ml) was added dropwise to a stirred solution of ethyl piperidine-4-carboxylate (30 g) in ethyl acetate (150 ml) which had been cooled to 0 to 5 C. in an ice-bath. The resultant mixture was stirred at ambient temperature for 48 hours. The mixture was poured into water (300 ml). The organic layer was separated, washed in turn with water (200 ml), 0.1N aqueous hydrochloric acid solution (200 ml), a saturated aqueous sodium bicarbonate solution (200 ml) and brine (200 ml), dried over magnesium sulphate and evaporated. There was thus obtained ethyl N-tert-butoxycarbonylpiperidine-4-carboxylate (48 g); NMR Spectrum: (CDCl3) 1.25 (t, 3H), 1.45 (s, 9H), 1.55-1.7 (m, 2H), 1.8-2.0 (d, 2H), 2.35-2.5 (m, 1H), 2.7-2.95 (t, 2H), 3.9-4.1 (br s, 2H), 4.15 (q, 2H). A solution of the material so obtained in THF (180 ml) was cooled at 0 C. and lithium aluminium hydride (1M solution in TBF; 133 ml) was added dropwise. The mixture was stirred at 0 C. for 2 hours. Water (30 ml) and 2N aqueous sodium hydroxide solution (10 ml) were added in turn and the mixture was stirred for 15 minutes. The resultant mixture was filtered through diatomaceous earth and the solids were washed with ethyl acetate. The filtrate was washed in turn with water and with brine, dried over magnesium sulphate and evaporated. There was thus obtained N-tert-butoxycarbonyl-4-hydroxymethylpiperidine (36.3 g); NMR Spectrum: (CDCl3) 1.05-1.2 (m, 2H), 1.35-1.55 (m, 10H), 1.6-1.8 (m, 2H), 2.6-2.8 (t, 2H), 3.4-3.6 (t, 2H), 4.0-4.2 (br s, 2H). 1,4-Diazabicyclo[2.2.2]octane (42.4 g) was added to a solution of N-tert-butoxycarbonyl-4-hydroxymethylpiperidine (52.5 g) in tert-butyl methyl ether (525 ml) and the mixture was stirred at ambient temperature for 15 minutes. The mixture was then cooled in an ice-bath to 5 C. and a solution of 4-toluenesulphonyl chloride (62.8 g) in tert-butyl methyl ether (525 ml) was added dropwise over 2 hours while maintaining the reaction temperature at approximately 0 C. The resultant mixture was allowed to warm to ambient temperature and was stirred for 1 hour. Petroleum ether (b.p. 60-80 C., 1L) was added and the precipitate was removed by filtration. The filtrate was evaporated to give a solid residue which was dissolved in diethyl ether. The organic solution was washed in turn with 0.5N aqueous hydrochloric acid solution, water, a saturated aqueous sodium bicarbonate solution and brine, dried over magnesium sulphate and evaporated. There was thus obtained N-tert-butoxycarbonyl-4-(4-toluenesulphonyloxymethyl)piperidine (76.7 g), NMR Spectrum: (CDCl3) 1.0-1.2 (m, 2H), 1.45 (s, 9H), 1.65 (d, 2H), 1.75-1.9 (m, 2H), 2.45 (s, 3H), 2.55-2.75 (m, 2H), 3.85 (d, 1H), 4.0-4.2 (br s, 2H), 7.35 (d, 2H), 7.8 (d, 2H). A portion (40 g) of the material so obtained was added to a suspension of <strong>[617-05-0]ethyl 4-hydroxy-3-methoxybenzoate</strong> (19.6 g) and potassium carbonate (28 g) in DMF (200 ml) and the resultant mixture was stirred and heated to 95 C. for 2.5 hours. The mixture was cooled to ambient temperature and partitioned between water and a mixture of ethyl acetate and diethyl ether. The organic layer was washed in turn with water and brine, dried over magnesium sulphate and evaporated. The resulting oil was crystallised from petroleum ether (b.p. 60-80 C.) and the suspension was stored overnight at 5 C. The resultant solid was collected by filtration, washed with petroleum ether and dried under vacuum. There was thus obtained ethyl 4-(N-tert-butoxycarbonylpiperidin-4-ylmethoxy)-3-methoxybenzoate (35 g), m.p. 81-83 C.; NMR Spectrum: (CDCl3) 1.2-1.35 (m, 2H), 1.4 (t, 3H), 1.48 (s, 9H), 1.8-1.9 (d, 2H), 2.0-2.15 (m, 2H), 2.75 (t, 2H), 3.9 (d, 2H), 3.95 (s, 3H), 4.05-4.25 (br s, 2H), 4.35 (q, 2H), 6.85 (d, 1H), 7.55 (s, 1H), 7.65 (d, 1H). The material so obtained was dissolved in formic acid (35 ml), formaldehyde (12M, 37% in water, 35. ml) was added and the mixture was stirred and heated to 95 C. for 3 hours. The resultant mixture was evaporated. The residue was dissolved in methylene chloride and hydrogen chloride (3M solution in diethyl ether; 40 ml) was added. The mixture was diluted with diethyl ether and the mixture was triturated until a solid was formed. The solid was collected, washed with diethyl ether and dried under vacuum overnight at 50 C. There was thus obtained ethyl 3-methoxy4(N-methylpiperidin-4-ylmethoxy)benzoate (30.6 g), NMR Spectrum: (DMSOd6) 1.29 (t, 3H), 1.5-1.7 (m, 2H), 1.95 (d, 2H), 2.0-2.15 (br s, 1H), 2.72 (s, 3H), 2.9-3.1 (m, 2H), 3.35-3.5 (br s, 2H), 3.85 (s, 3H), 3.9-4.05 (br s, 2H), 4.3 (q, 2H), 7.1 (d, 1H), 7.48 (s, 1H), 7.6 (d, 1H). The material so obtained was dissolved in methylene chloride (75 ml) and the solution was cooled in an ice-bath to 0-5 C. Trifluoroacetic acid (37.5 ml) was added followed by the dropwise addition over 15 minutes... |
|
With potassium carbonate; In DMF (N,N-dimethyl-formamide); at 95℃; for 2.5h; |
A portion (40 g) of the material so obtained was added to a suspension of <strong>[617-05-0]ethyl 4-hydroxy-3-methoxybenzoate</strong> (19.6 g) and potassium carbonate (28 g) in DMF (200 ml) and the resultant mixture was stirred and heated to 95 C. for 2.5 hours. The mixture was cooled to ambient temperature and partitioned between water and a mixture of ethyl acetate and diethyl ether. The organic layer was washed in turn with water and brine, dried over magnesium sulphate and evaporated. The resulting oil was crystallised from petroleum ether (b.p. 60-80 C.) and the suspension was stored overnight at 5 C. The resultant solid was collected by filtration, washed with petroleum ether and dried under vacuum. There was thus obtained ethyl 4(N-tert-butoxycarbonylpiperidin-4-ylmethoxy)-3-methoxybenzoate (35 g), m.p. 81-83 C.; NMR Spectrum: (CDCl3) 1.2-1.35 (m, 2H), 1.4 (t, 3H), 1.48 (s, 9H), 1.8-1.9 (d, 2H), 2.0-2.15 (m, 2H), 2.75 (t, 2H), 3.9 (d, 2H), 3.95 (s, 3H), 4.05-4.25 (br s, 2H), 4.35 (q, 2H), 6.85 (d, 1H), 7.55 (s, 1H), 7.65 (d, 1H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping


 from Mycobacterium tuberculosis.png)