| 100% |
|
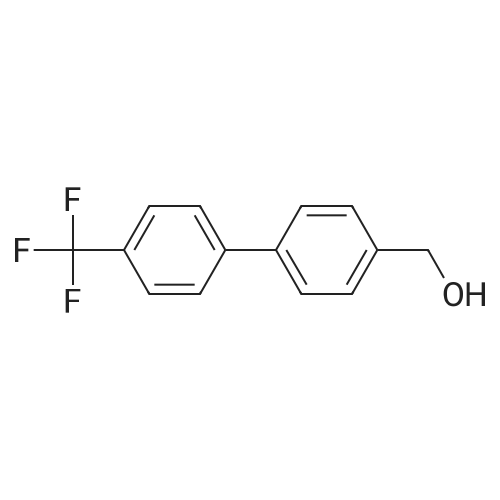
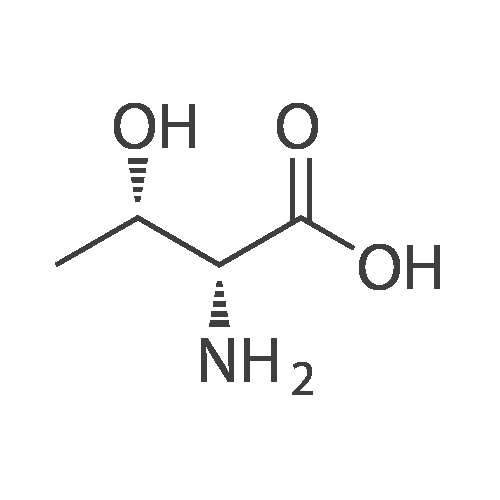
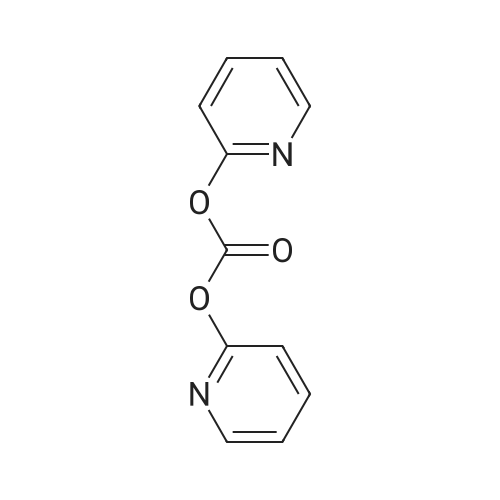
Under nitrogen atmosphere, to a stirred mixture of <strong>[457889-46-2][4-[4-(trifluoromethyl)-phenyl]-phenyl]-methanol</strong> (0.3 g, 1.19 mmol) in dry CH2Cl2 (2.0 mL), DMAP (0.015 g, 0.12 mmol) and di-2-pyridyl-carbonate (0.309 g, 1.43 mmol) were added. The reaction mixture was left to react at rt for 15 h, then diluted with CH2Cl2 and washed first with a saturated NH4Cl solution (3.0 mL) and subsequently with a saturated NaHCO3 solution (3×3 mL). The organic fraction was dried over Na2SO4, filtered and concentrated to dryness to afford a colorless oil (0.3 g, 68%), as a mixture (ratio 1.8:1) of 2-pyridyl-[4-[4-(trifluoromethyl)-phenyl]-phenyl]-methyl carbonate and [4-[4-(trifluoromethyl)-phenyl]-phenyl]-methyl-2-oxopyridine-1-carboxylate. The mixture of isomers was not separated and used in the next step without any further purification. To a stirred mixture of D-threonine (0.063 g, 0.53 mmol) and NaHCO3 (0.067 g, 0.8 mmol) in H2O (3.0 mL), the crude mixture containing 2-pyridyl-[4-[4-(trifluoromethyl)-phenyl]-phenyl]-methyl carbonate and [4-[4-(trifluoromethyl)-phenyl]-phenyl]-methyl-2-oxopyridine-1-carboxylate (0.3 g, 0.8 mmol) in THF (3.0 mL) was added. After 15 h at rt, the crude mixture was rotary evaporated to remove the organics and subsequently extracted with Et2O (3×5 mL). The aqueous phase was acidified with 2.0 M HCl solution to pH 2-3 and subsequently extracted with EtOAc (3×10 mL). The organic fraction was dried over Na2SO4, filtered and concentrated to dryness to afford the title compound as transparent oil (0.21 g, quant.), which was used in the next step without further purification. MS (ESI) m/z: 415 [M-NH4]+; (ESI) m/z: 396 [M-H]-. 1H NMR (DMSO-d6): delta 1.11 (d, J=6.4 Hz, 3H), 3.97 (dd, J=3.5, 8.9 Hz, 1H), 4.05-4.12 (dq, J=3.5, 6.4 Hz, 1H), 5.13 (s, 2H), 7.00 (d, J=8.9 Hz, 1H), 7.51 (d, J=8.1 Hz, 2H), 7.75 (d, J=8.1 Hz, 2H), 7.82 (d, J=8.1 Hz, 2H), 7.91 (d, J=8.1 Hz, 2H), 12.59 (s, 1H). |
| 0.21 g |
|
To a stirred mixture of D-threonine (0.063 g, 0.53 mmol) and NaHC03 (0.067 g, 0.8 mmol) in H20 (3.0 mL), the crude mixture containing 2-pyridyl-[4-[4-(trifluoromethyl)- phenyl]-phenyl]-methyl carbonate and [4-[4-(trifluoromethyl)-phenyl]-phenyl]-methyl-2- oxopyridine-l-carboxylate (0.3 g, 0.8 mmol) in THF (3.0 mL) was added. After 15 h at rt, the crude mixture was rotary evaporated to remove the organics and subsequently extracted with Et20 (3x5 mL). The aqueous phase was acidified with 2.0 M HC1 solution to pH 2-3 and subsequently extracted with EtOAc (3x10 mL). The organic fraction was dried over Na2S04, filtered and concentrated to dryness to afford the title compound as transparent oil (0.21 g, quant.), which was used in the next step without further purification. MS (ESI) m/z: 415 [M- NH4]+; (ESI) m/z: 396 [M-H]-. 1H NMR (DMSO-d6): delta 1.1 1 (d, J= 6.4 Hz, 3H), 3.97 (dd, J= 3.5, 8.9 Hz, 1H), 4.05 - 4.12 (dq, J= 3.5, 6.4 Hz, 1H), 5.13 (s, 2H), 7.00 (d, J= 8.9 Hz, 1H), 7.51 (d, J= 8.1 Hz, 2H), 7.75 (d, J= 8.1 Hz, 2H), 7.82 (d, J= 8.1 Hz, 2H), 7.91 (d, J= 8.1 Hz, 2H), 12.59 (s, 1H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping