|
|
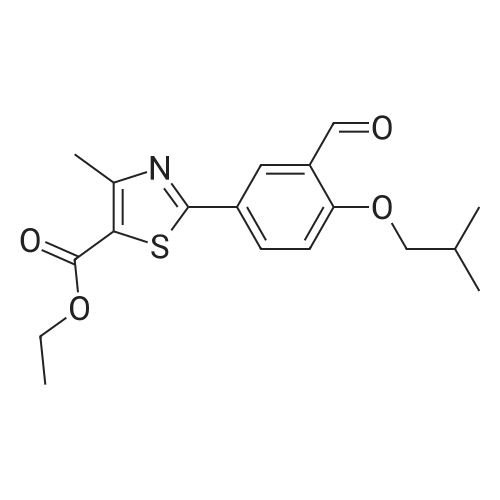
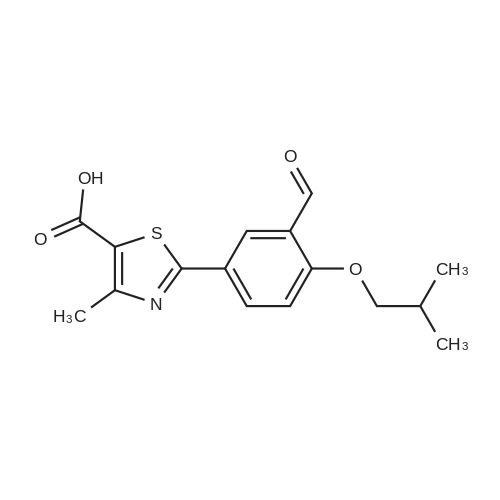
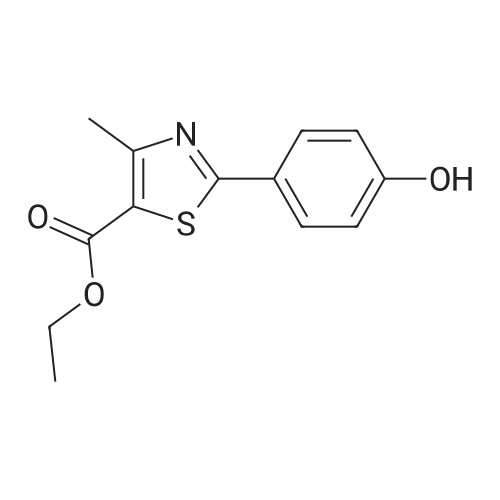
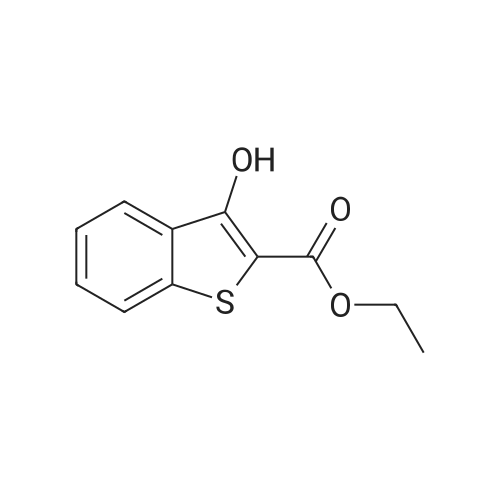
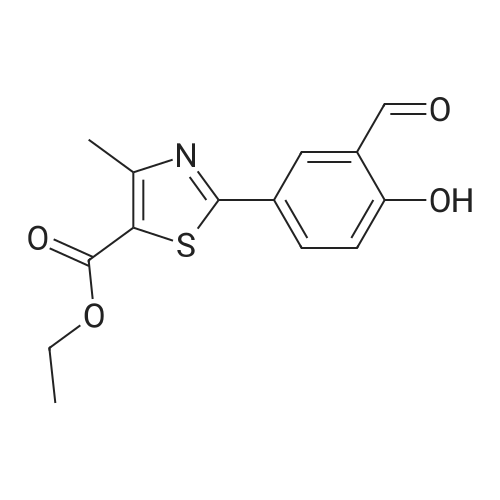
To a stirred solution of ethyl 2-[3-formyl-4-hydroxyphenyl]-4-methylthiazole-5- carboxylate (10 gms) in dimethylformamide (40 ml) added potassium carbonate (9.78 gms) and potassium iodide (2.35 gms). Heated the reaction mixture to 70 - 75C and stirred for 30 minutes. To the above reaction added a solution of l-bromo-2- methylpropane (9.72 gms) in dimethyl formamide (10 ml) and stirred for 5 hours. Cooled the reaction mixture to 25C, quenched with water and stirred for one hour. Filtered the precipitated solid and dried the material to get the title compound. Yield: 9 gms. |
|
With potassium carbonate; N,N-dimethyl-formamide; at 87 - 93℃; for 4h; |
b) Preparation of Ethyl 2-(3-formyl-4-isobutoxyphenyl)-4-methyl-5-thiazolecarboxylate [Compound of formula IV1.350.0gm of Ethyl 2-(3-formyl-4-hydroxyphenyl)-4-methyl-5-thiazolecarboxylate, [Compound of formula III] 332. Ogm of potassium carbonate and 330.0gm of isobutyl bromide were added to 1.751tr of DMF. Reaction mixture was heated to 90 +/- 3C and stirred for 4 hr. Reaction mixture was cooled to 25C and slowly added 10.50 ltr of water. Slurry of the product formed was stirred for 2.0hr, filtered, washed and dried under vacuum to give 389 gm of titled compound.Analytical Data- · ^NMR (CDC13, 400 MHz) : delta 1.079-1.101 (doublet, 6H); delta 1.366-1.413 (triplet,3H); delta 2.185-2.230 (multiplet, 1H); delta 2.769 (singlet, 3H); delta 3.914-3.935 (doublet, 2H); delta 4.316-4.387 (quartet, 2H); delta 7.045-7.074 (doublet, 1H); delta 8.188-8.225 (doublet of doublet, 1H); delta 8.353-8.361 (doublet, 1H).? Mass (m/e): 348.3 |
|
With potassium carbonate; potassium iodide; In N,N-dimethyl-formamide; at 25 - 85℃; for 5.08333h; |
Example 3:Preparation of 2-(3-formyl-4-isobutoxyphenyI)-4-methyIthiazole-5-carboxylic acid ethyl esterTo a dimethylformamide (1250 ml) was added 2-(3-formyl-4-hydroxyphenyl)-4- methylthiazole-5-carboxylic acid ethyl ester (250 gm) at room temperature for 5 minutes to obtain a solution. To the solution was added potassium carbonate (250 gm) and potassium iodide (62.5 gm), and then added isobutyl bromide (100 gm) slowly for 2 hours. The temperature of the reaction mass was raised to 85C and maintained for 3 hours. The dimethylformamide solvent was distilled off under vacuum at 80C and then cooled to room temperature. The reaction mass was added water (2500 ml) and stirred for 2 hours at room temperature. The separated solid was filtered, washed with cycloheaxne and then dried to obtain 285 gm of 2-(3-formyl-4-isobutoxyphenyl)-4-methylthiazole-5- carboxylic acid ethyl ester. |
| 16.43 g |
With potassium carbonate; In N,N-dimethyl-formamide; at 20 - 80℃; for 4h; |
Dissolve 14.14g of <strong>[161798-01-2]ethyl 2-(3-formyl-4-hydroxyphenyl)-4-methylthiazole-5-carboxylate</strong> (Formula III) in 55 ml dimethylformamide, at ambient temperature. Add 40g of potassium carbonate, along with 15.9 ml isobutyl bromide. Heat the reaction to 75-80 C and stir for 4 hours. Cool to 25-30 C, while 165 ml process water is added. Further cool to 0-5 C and stir for 30 minutes at this temperature. Filter off the precipitated solid and wash the filter cake with 55 ml process water. The wet cake is dried under vacuum at 40 C for 7 hours, to furnish 16.43 g of ethyl 2-(3-formyl-4-isobutoxyphenyl)-4-methylthiazole-5-carboxylate (Formula lib). of compound of formula Illb |
| 34.2 g |
With potassium carbonate; In N,N-dimethyl-formamide; at 60 - 115℃; |
Formylation reaction is complete, no need to separate,Continue the next isobutylation reaction,DMF as the reaction solvent,164g of dimethylformamide was added, dissolved,Then add 14g potassium carbonate,21.7g bromoisobutane was added dropwise, the reaction was warmed,The reaction temperature is controlled at 60-115 C,The reaction time is 3-10 hours, the reaction was completely detected by HPLC,Slowly dropping 76g of water,Cooled to room temperature filtered, centrifuged,Centrifuge the filter cake first with 50g DMF (dimethylformamide) once, high-speed spin-dry;Then washed with 19g of water for the first time,High-speed centrifuge drying, washing with 19g water a second time,High-speed centrifuge drying;Finally, the first wash with 15g methanol,High-speed centrifuge drying, washed with 15g methanol second time,High-speed centrifuge drying;The wet product was dried to give 34.2 g of ethyl 2- (3-aldehyde-4-isobutyloxyphenyl) -4-methylthiazole-5-Chromatographic purity of 99.2%Content of 99.5%, two-step total yield of 68.2%. |
|
With triethylamine; at 75℃; for 15h;Large scale; |
Add 400 kg of DMAC, 100 kg of 2- (3-formyl-4-hydroxyphenyl) -4-methylthiazole-5-carboxylic acid ethyl ester to the reaction tank,50kg bromoisobutane, 35kg triethylamine,The temperature was raised to 75 C for 15h.Then, 25 kg of hydroxylamine hydrochloride was added at a reduced temperature, and reacted at 75 C for 3 hours.Then 30 kg of acetyl chloride was added dropwise and reacted at 80 C for 7 hours.After the reaction was completed, the temperature was lowered to 20-30 C, water was added, 20 kg of sodium hydroxide was added, and hydrolysis was performed at 45 C for 6 hours.Then add hydrochloric acid dropwise to adjust the pH to 6.0-7.0. Reduce the temperature to 0-5 C, then add water and crystallize for 2h.After centrifugation, a white solid powder was obtained, and the obtained powder was crude febuxostat with a wet weight of 128 kg. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping