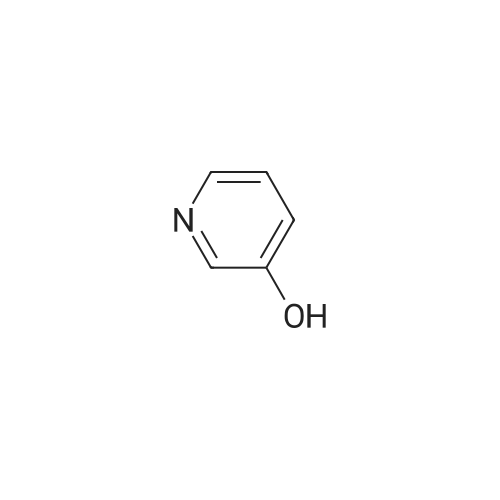
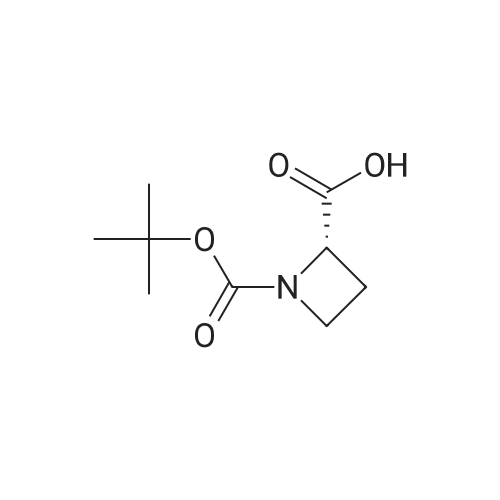
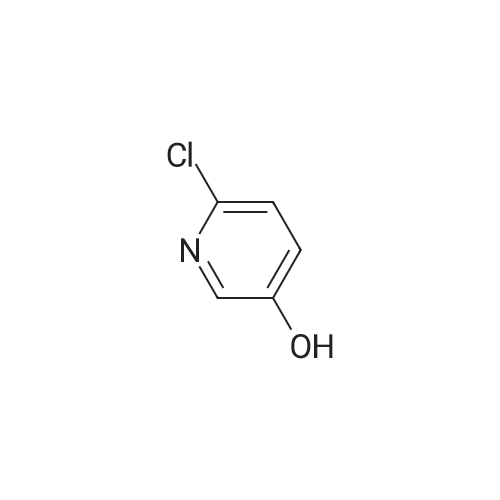
| 85% |
With triphenylphosphine; diethylazodicarboxylate; In tetrahydrofuran; at 20℃; for 48h; |
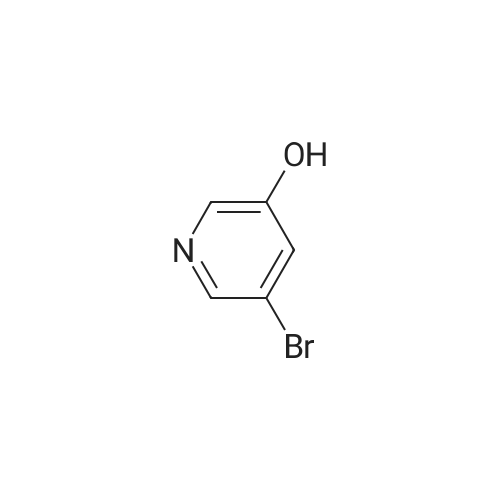
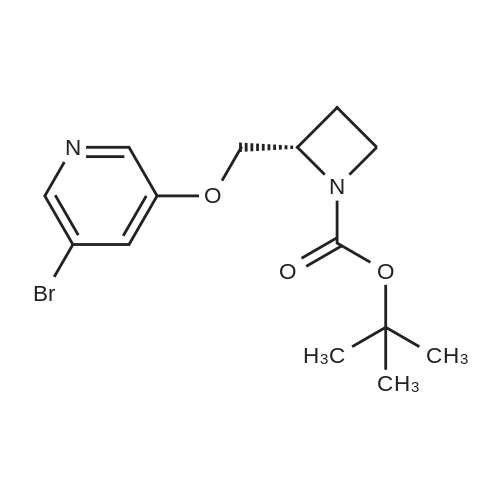
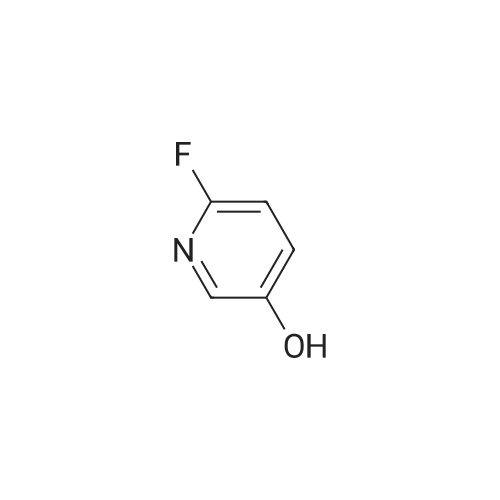
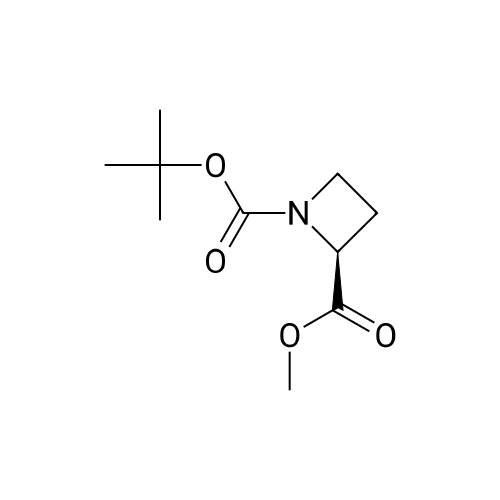
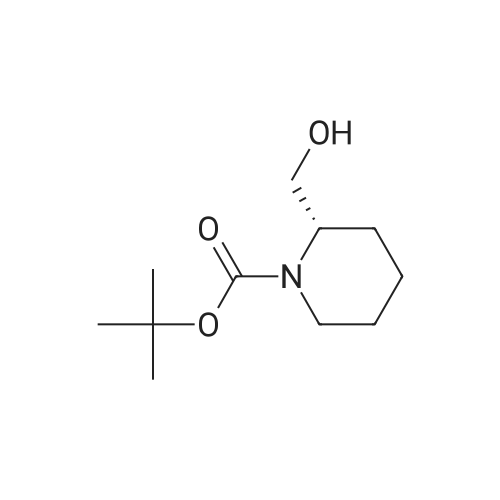
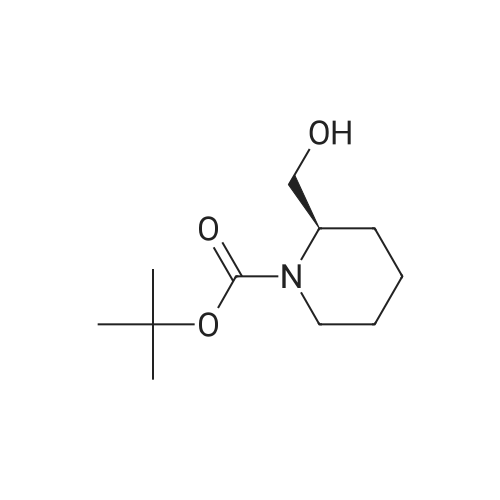
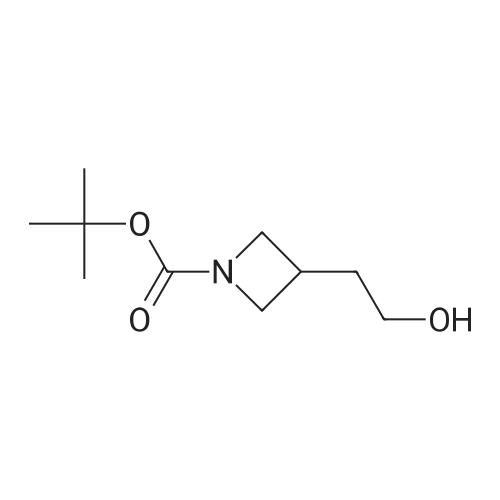
To a stirred solution of 1-tert-butoxycarbonyl-2 (5)-AZETIDINE (800 mg, 4.3 mmol) and 3-bromo-5- hydroxypyridine (800 mg, 4.6 mmol), and PPh3 (1.69 g, 6.45 mmol) in THF (50 mL) was slowly added DEAD (1.02 mL, 6.45 mmol). The reaction mixture was stirred at room temperature for 48 h, and concentrated in vacuo. The residue was purified by chromatography with hexane-EtOAc (4: 1) to give a light yellow oil (1.25 g, 85%). H NMR (CDC13) 6 8.29 (d, 1H, J= 2.1 Hz), 8. 28 (d, 1H, J= 2.7 Hz), 7.43 (t, 1H, J= 2.4 Hz), 4.51 (M, 1H), 4.34 (M, 1H), 4. 13 (dd, 1H, J= 10.2, 3.0 Hz), 3. 89 (t, 2H, J= 7.5 Hz), 2.42-2. 22 (M, 2H), 1.43 (s, 9H) ; 13C NMR (CDC13) 8 156.05, 155.35, 143.07, 136.58, 123.96, 120.25, 79.75, 68. 90,59. 84, 47.02, 28. 31,18. 88. |
| 70% |
With triphenylphosphine; diethylazodicarboxylate; In tetrahydrofuran; |
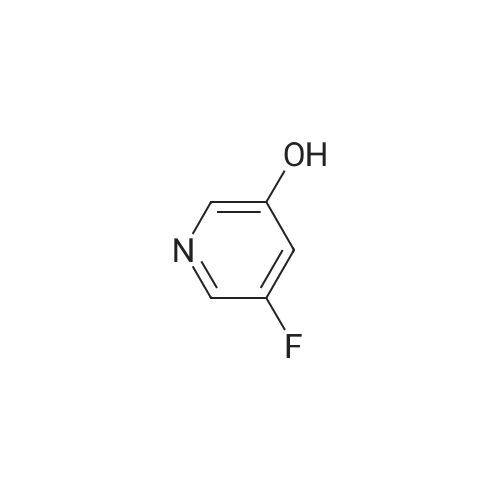
A gram-scale synthesis of sazetidine A is outlined in Figure Ia. The Boc-protected azidityl methyl alcohol 2 and 3 -bromo-5 -hydroxy pyridine (3) are both commercially available. Treatment with PPh3 and DEAD according to the Mitsunobu protocol formed ether derivative 4 in 70% yield. Palladium-mediated coupling of the bromo-substituted pyridine 4 with 5-hexyn-l-ol under modified Sonogashira conditions furnished Boc- protected sazetidine A in 73% yield. Deprotection of the Boc-group with HCl was expected to give sazetidine A in salt form as published in the literature. However, after several <n="43"/>AtIy Docket No.: GUX-023.25 attempts, it was found that the HCl salt, especially on larger scale, was a white solid that could be obtained in only moderate yield by a tedious filtration under an inert atmosphere. Exposure of the material to air immediately resulted in decomposition of the material to a yellow-brown wet solid. Therefore, in order to obtain gram quantities of pure sazetidine A, it was found that it was necessary to treat the deprotected material immediately with NH4OH and then isolated the free base of sazetidine A. This product was then taken up in an aqueous solution of HCl to give a solution of the HCl salt of sazetidine A. This modified method gives purer material and was indeed found to be more potent than that reported in the literature. |
| 64% |
With triphenylphosphine; diethylazodicarboxylate; In tetrahydrofuran; toluene; at 0 - 20℃;Inert atmosphere; |
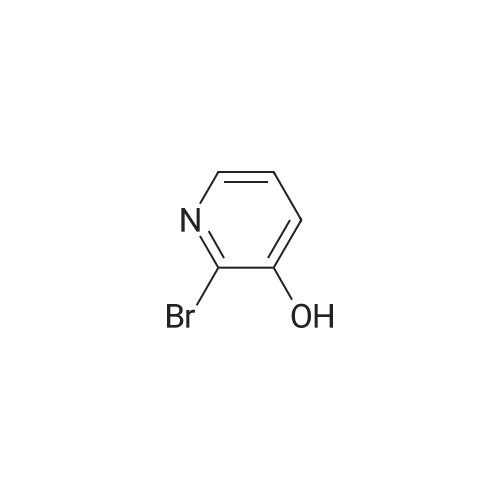
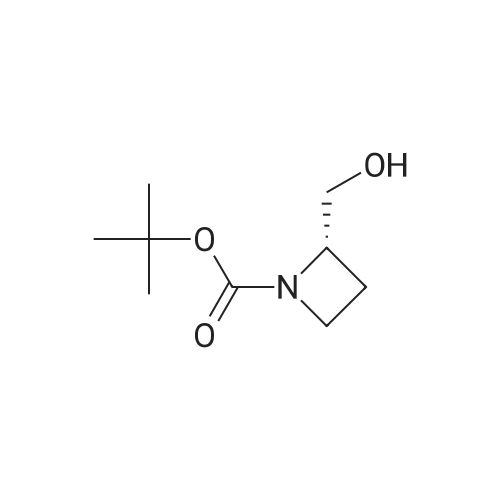
Under N2 stream, triphenylphosphine (7.8 g, 29.7 mmol), 40% diethyl azodicarboxylate (40% solution in toluene, 5.2 mL), and 5-bromo-3-hydroxynicotinic acid (5.2 g, 29.7 mmol) at 0 C were added to a solution of 1 (3.6 g, 19.8 mmol) in THF (20 mL). The mixture was stirred at 0 C for 10 min and then warmed to room temperature and stirred overnight. After the reaction, the solvent was removed under reduced pressure followed by silica gel chromatography (petroleum ether/ethylacetate=1/1) to yield 8 (4.35 g, 12.7 mmol, 64%) as a colorless oil. 1HNMR (500 MHz, CDCl3) δ; 8.29 (t, J=2.6 Hz, 2H), 7.45-7.42 (m, 1H),4.55-4.48 (m, 1H), 4.33 (broad s, 1H), 4.13 (dd, J=7.5, 2.9 Hz, 1H),3.89 (m, 2H), 2.39-2.24 (m, 2H), 1.43 (s, 9H). 13C NMR (125 MHz,CDCl3) δ; 156.1, 155.4, 143.2, 136.7, 124.1, 120.3, 79.9, 69.0, 64.2,59.5, 28.4, 19.0. MS (EI+) m/z; 344 ([M+2]+, 1.97), 342 (M+, 1.92),271 (5.59), 269 (5.81), 243 (5.83), 241 (6.09), 156 (28), 113 (22), 100(34), 57 (1 0 0). HRMS (EI+) m/z; 342.0585 (Calcd: 342.0578 forC14H19N2O3Br). [α]D20=-59.63 (c=2.24, CHCl3). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping