Investigating the replacement of carboxylates with carboxamides to modulate the safety and efficacy of platinum(II) thioether cyanide scavengers
Behymer, Matthew M
;
Mo, Huaping
;
Fujii, Naoaki
, et al.
Toxicol. Sci.,2024,197(2):197-210.
DOI:
10.1093/toxsci/kfad119
PubMed ID:
37952247
More
Abstract: Cyanide represents a persistent threat for accidental or malicious misuse due to easy conversion into a toxic gas and access to large quantities through several industries. The high safety index of hydroxocobalamin is a cornerstone quality as a cyanide scavenger. Unfortunately, intravenous infusion of hydroxocobalamin limits the utility in a mass casualty setting. We previously reported platinum(II) [Pt(II)] complexes with trans-directing sulfur ligands as an efficacious alternative to hydroxocobalamin when delivered by a bolus intramuscular injection in mice and rabbits. Thus, to enable Pt(II) as an alternative to hydroxocobalamin, a high safety factor is needed. The objective is to maintain efficacy and mitigate the risk for nephrotoxicity. Platinum amino acid complexes with the ability to form five- or six-membered rings and possessing either carboxylates or carboxamides are evaluated in vitro for cyanide scavenging. In vivo efficacy was evaulated in the zebrafish and mice cyanide exposure models. In addition, Pt(II) complex toxicity and pharmacokinetics were evaluated in a cyanide naive Sprague-Dawley model. Doses for toxicity are escalated to 5x from the efficacious dose in mice using a body surface area adjustment. The results show the carboxamide ligands display a time and pH dependence on cyanide scavenging in vitro and efficacy in vivo. Additionally, exchanging the carboxylate for carboxamide showed reduced indications of renal injury. A pharmacokinetic analysis of the larger bidentate complexes displayed rapid absorption by intramuscular administration and having similar plasma exposure. These findings point to the importance of pH and ligand structures for methionine carboxamide complexes with Pt(II).
Keywords:
exposures ;
countermeasure ;
cyanide ;
pharmacokinetics ;
chemical threat ;
safety and efficacy
Purchased from AmBeed:
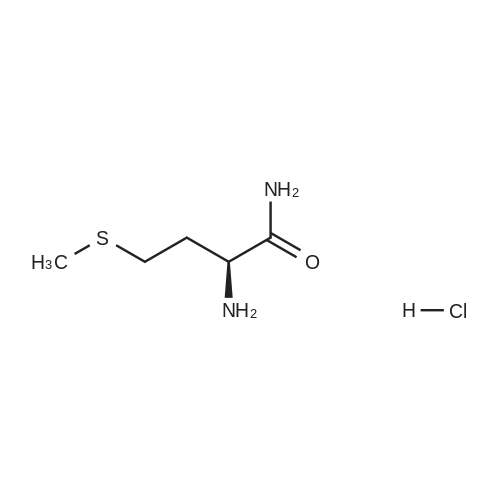
16120-92-6 ;
36499-65-7
 thioether cyanide scavengers.png)
Identification of Platinum(II) Sulfide Complexes Suitable as Intramuscular Cyanide Countermeasures
Behymer, Matthew M.
;
Mo, Huaping
;
Fujii, Naoaki
, et al.
Chem. Res. Toxicol.,2022,35(11):1983-1996.
DOI:
10.1021/acs.chemrestox.2c00157
PubMed ID:
36201358
More
Abstract: The development of rapidly acting cyanide countermeasures using i.m. injection (IM) represents an unmet medical need to mitigate toxicant exposures in mass casualty settings. Previous work established that cisplatin and other platinum(II) or platinum(IV)-based agents effectively mitigate cyanide toxicity in zebrafish. Cyanide′s in vivo reaction with platinum-containing materials was proposed to reduce the risk of acute toxicities. However, cyanide antidote activity depended on a formulation of platinum-chloride salts with DMSO (DMSO) followed by dilution in phosphate-buffered saline (PBS). A working hypothesis to explain the DMSO requirement is that the formation of platinum-sulfoxide complexes activates the cyanide scavenging properties of platinum. Preparations of isolated NaPtCl5-DMSO and Na (NH3)2PtCl-DMSO complexes in the absence of excess DMSO provided agents with enhanced reactivity toward cyanide in vitro and fully recapitulated in vivo cyanide rescue in zebrafish and mouse models. The enhancement of the cyanide scavenging effects of the DMSO ligand could be attributed to the activation of platinum(IV) and (II) with a sulfur ligand. Unfortunately, the efficacy of DMSO complexes was not robust when administered IM. Alternative Pt(II) materials containing sulfide and amine ligands in bidentate complexes show enhanced reactivity toward cyanide addition The cyanide addition products yielded tetracyanoplatinate(II), translating to a stoichiometry of 1:4 Pt to each cyanide scavenger. These new agents demonstrate a robust and enhanced potency over the DMSO-containing complexes using IM administration in mouse and rabbit models of cyanide toxicity. Using the zebrafish model with these Pt(II) complexes, no acute cardiotoxicity was detected, and dose levels required to reach lethality exceeded 100 times the ED. Data are presented to support a general chem. design approach that can expand a new lead candidate series for developing next-generation cyanide countermeasures.
Purchased from AmBeed:
65-82-7 ;
15663-27-1 ;
18542-42-2 ;
1187-84-4 ;
63-68-3 ;
16120-92-6 ;
4104-45-4
 Sulfide Complexes Suitable as Intramuscular Cyanide Countermeasures.png)

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping


 thioether cyanide scavengers.png)
 Sulfide Complexes Suitable as Intramuscular Cyanide Countermeasures.png)
