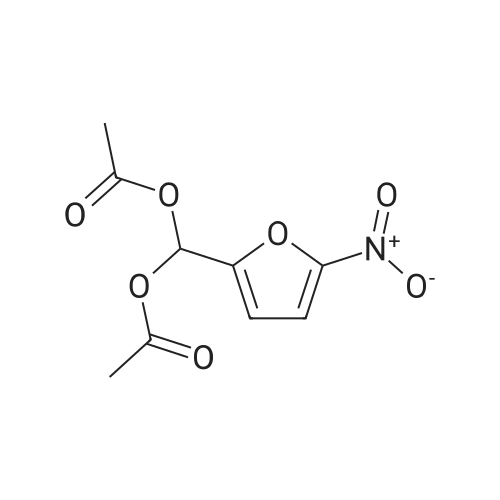
| 96% |
With trichlorophosphate; In acetonitrile; at 75℃; for 1.25h;Inert atmosphere; |
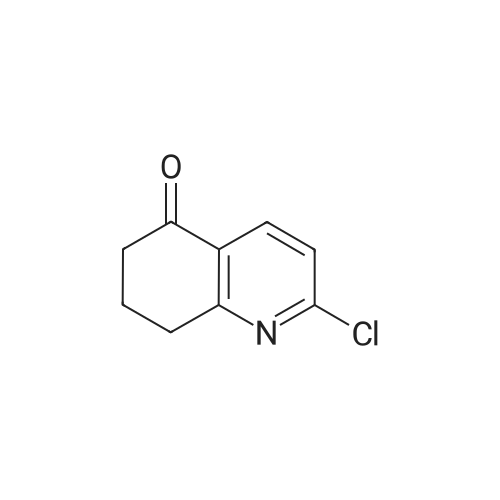
Example 3A 2-Chloro-7,8-dihydroquinolin-5(6H)-one Under nitrogen, 21.02 g (0.13 mol) of 7,8-dihydroquinoline-2,5(1H,6H)-dione were suspended in 100 ml of acetonitrile (anhydrous, <30 ppm H2O), and 135.28 ml (density 1.46 g/ml, 1.29 mol) of phosphorus oxychloride were added. The yellowish suspension was then heated to 75 C. and stirred at this temperature for 1.25 hours. The yellow clear solution was then cooled to room temperature, and 150 ml of toluene were added. The solution was then concentrated on a rotary evaporator to about 100 ml, and another 150 ml of toluene were added. The solution was then concentrated to dryness on a rotary evaporator. 300 ml of ethyl acetate were then added to the orange oil obtained. Subsequently, the solution was carefully (evolution of gas) added to 500 ml of saturated aqueous sodium bicarbonate solution and stirred for 15 min. The phases were separated and the aqueous phase was extracted with 200 ml of ethyl acetate. The combined organic phases were washed twice with 250 ml of water and once with 100 ml of saturated sodium chloride solution, dried over sodium sulphate, filtered and concentrated to dryness under reduced pressure. This gave 22.58 g (0.12 mmol, 96% of theory) of the target compound as a slightly yellowish solid. 1H-NMR (400 MHz, DMSO-d6, delta/ppm): 2.06-2.17 (m, 2H), 2.61-2.70 (m, 2H), 3.05 (t, 2H), 7.51 (d, 1H), 8.18 (d, 1H). |
| 90.9% |
With trichlorophosphate; In acetonitrile; for 2h;Reflux; |
To a solution of 7,8-dihydroquinoline-2,5(1H,6H)-dione (5 g, 27.6 mmol ) in acetonitrile(50 mL) was added POC13 (12.6 g, 82.5 mmol) dropwise at 0 C. The cooling bath was removedand the reaction mixture was refluxed for 2h. The reaction mixture was cooled to room temperature and the volatiles were evaporated under reduced pressure to get the residue, which was neutralized with ammonium hydroxide and extracted with ethyl acetate. The organic layer was dried over sodium sulfate, filtered and concentrated to get the title compound (5 g, 90.9%).LC-MS: 181.9 [M+H]. |
| 74% |
|
3.2 2-Chloro-7,8-dihydroquinolin-5(6H)-oneTo a suspension of 7,8-dihydroquinoline-2,5(1H,6H)-dione (1.5 g, 9.19 mmol) in acetonitrile (22 mL) was added dropwise phosphorous oxychloride (1.714 mL, 18.39 mmol). The resulting solution was heated to 100 C. and stirred for 2 h. The reaction was cooled to RT and poured into ice-cold water. After basifying the mixture with 2 M sodium hydroxide solution it was extracted with ethyl acetate (3×). After each extraction the pH of the aqueous phase was checked and if necessary adjusted by adding 1 M sodium hydroxide solution. The combined organic layers were dried over sodium sulfate, filtered, and evaporated to dryness. The crude was purified by flash chromatography (silica gel, cyclohexane/ethyl acetate) yielding a colourless solid. Amount 1.23 g. Yield 74%.1H-NMR (DMSO-d6, 400 MHz) delta 2.13 (m, 2H), 2.68 (m, 2H), 3.08 (t, 2H), 7.53 (d, 1H), 8.20 (d, 1H)MS (ES-API) m/z 182.0 (M+H+, 100%). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping