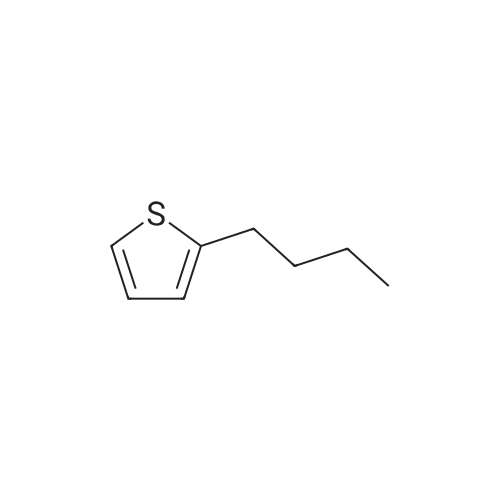
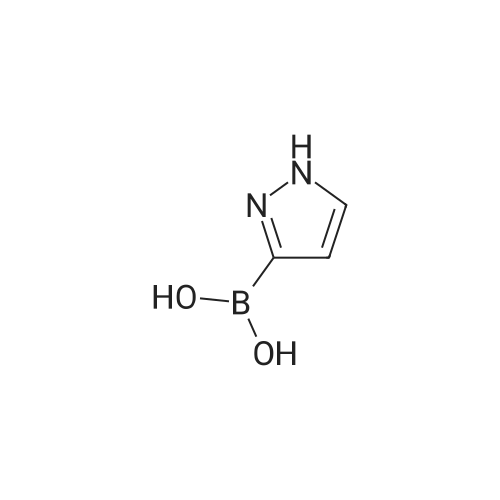
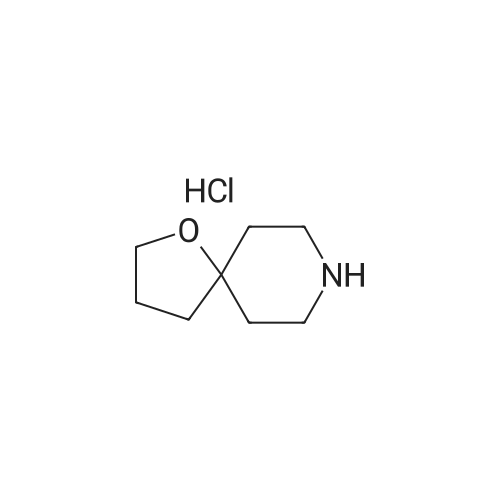
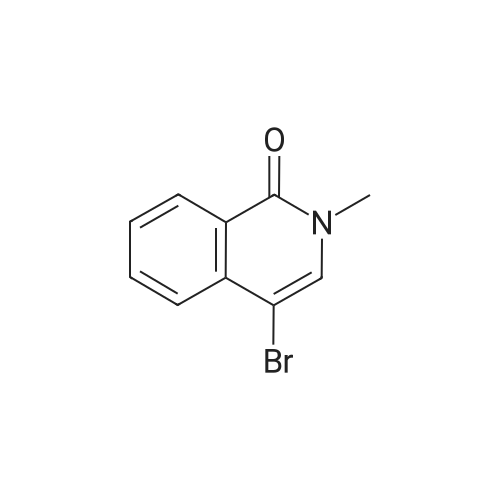
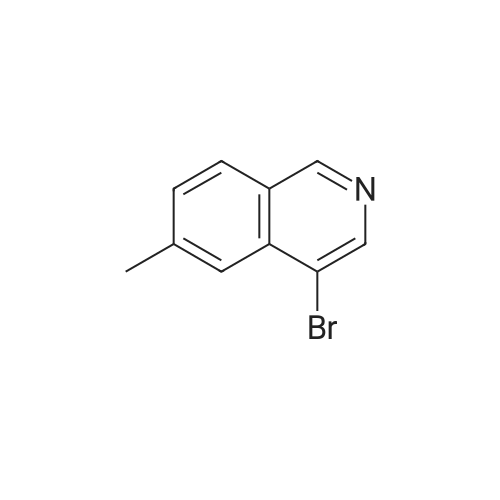
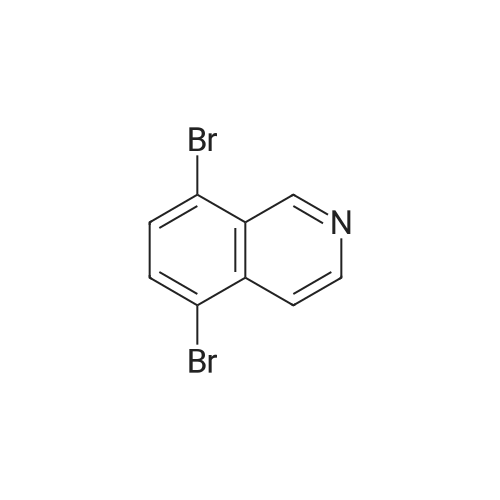
| 60% |
|
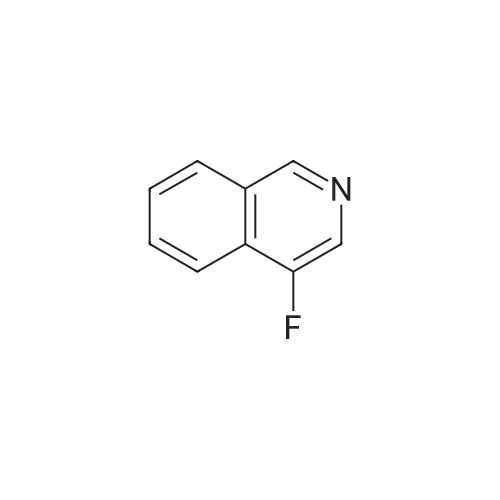
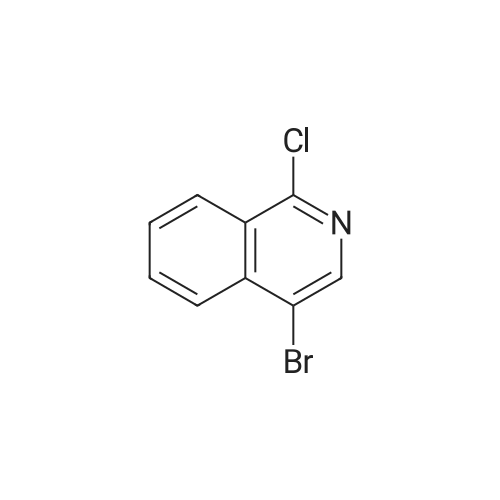
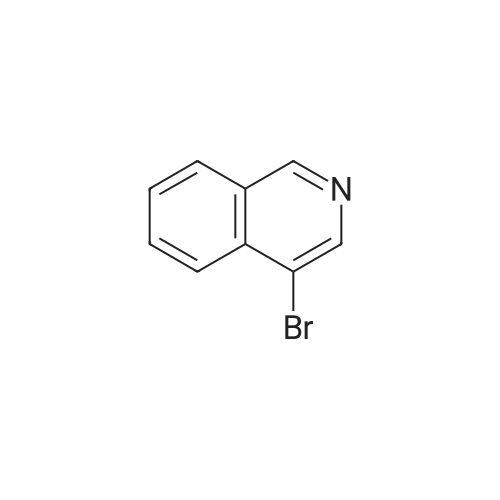
To a solution of n-butyl lithium (133 mL, 332.5 mmol, 2.5 M in THF) in 760 mL THF was added a solution of 4-bromoisoquinoline 24a (20 g, 96.6 mmol) in 144 mL THF dropwise at -65° C. and the resulting mixture was stirred at this temperature for another 30 mm after the completion of addition. A solution of N-fluorobenzenesulfonimide (66.68 g, 211.7 mmol) in 216 mL THF was added at -65° C. in 1 h dropwisely. After being stirred for another 1 h at this temperature, the reaction mixture was warmed to room temperature slowly with stirring, after the reaction is over, 300 mL saturated aq. NH4Cl was added slowly, extracted with EtOAc (300 mL*3). The combined organic layers were washed with 300 mL brine, dried over anhydrous Na2SO4 and concentrated. The crude product was purified by silica gel column chromatography (0-100percent EtOAc/PE) to give 4-fluoroisoquinoline 24b (8.5 g, red oil, yield: 60percent). |
|
|
Reference Example 1 4-Fluoroisoquinoline A solution of n-butyllithium in n-hexane (1.58 M, 60.1 ml, Kanto Chemicals) was added with tetrahydrofuran (345 ml), and the mixture was sufficiently cooled on a dry ice-acetone bath. The mixture was added dropwise with a solution of 4-bromoisoquinoline (9.0 g, Tokyo Kasei Kogyo) in tetrahydrofuran (65 ml) over 1 hour so that the temperature of the reaction mixture should not exceed -65° C. The mixture was stirred at the same temperature for 30 minutes, and then added dropwise with a solution of N-fluorobenzenesulfonimide (30 g, Tokyo Kasei Kogyo) in tetrahydrofuran (100 ml) over 1 hour so that the temperature of the reaction mixture should not exceed -65° C. Subsequently, the mixture was stirred at the same temperature for 1 hour, then the cooling bath was removed, and the mixture was gradually warmed to room temperature. The reaction mixture was added with saturated aqueous sodium hydrogencarbonate (300 ml) and ethyl acetate (300 ml), and stirred at room temperature for 12 hours. The organic layer was separated, and the aqueous layer was extracted with ethyl acetate 3 times (200 ml for each time). The combined organic layer was washed with saturated brine (500 ml), and dried over anhydrous magnesium sulfate. The solvent was evaporated under reduced pressure, the residue was added with chloroform (250 ml), and the insoluble matters were removed by filtration. The solvent was evaporated under reduced pressure, and the residue was purified by silica gel chromatography (n-hexane:ethyl acetate=5:1) to obtain the title compound (3.6 g). MS (m/z): 148 (MH+) 1H-NMR (CDCl3) delta (ppm): 7.26-7.71 (1H, m), 7.75-7.82 (1H, m), 8.03 (1H, dd, J=1.2 Hz, J=8.4 Hz), 8.10 (1H, d, J=8.4 Hz), 8.38 (1H, s), 9.08 (1H, s) |
|
|
A solution of n-butyllithium in n-hexane (1.58 M, 60.1 ml, Kanto Chemicals) was added with tetrahydrofuran (345 ml), and the mixture was sufficiently cooled on a dry ice-acetone bath. The mixture was added dropwise with a solution of 4-bromoisoquinoline (9.0 g, Tokyo Kasei Kogyo) in tetrahydrofuran (65 ml) over 1 hour so that the temperature of the reaction mixture should not exceed -65° C. The mixture was stirred at the same temperature for 30 minutes, and then added dropwise with a solution of N-fluorobenzenesulfonimide (30 g, Tokyo Kasei Kogyo) in tetrahydrofuran (100 ml) over 1 hour so that the temperature of the reaction mixture should not exceed -65° C. Subsequently, the mixture was stirred at the same temperature for 1 hour, then the cooling bath was removed, and the mixture was gradually warmed to room temperature. The reaction mixture was added with saturated aqueous sodium hydrogencarbonate (300 ml) and ethyl acetate (300 ml), and stirred at room temperature for 12 hours. The organic layer was separated, and the aqueous layer was extracted with ethyl acetate 3 times (200 ml for each time). The combined organic layer was washed with saturated brine (500 ml), and dried over anhydrous magnesium sulfate. The solvent was evaporated under reduced pressure, the residue was added with chloroform (250 ml), and the insoluble solids were removed by filtration. The solvent was evaporated under reduced pressure, and the residue was purified by silica gel chromatography (n-hexane:ethyl acetate=5:1) to obtain the title compound (3.6 g).MS (m/z): 148 (MH+)1H-NMR (CDCl3) delta (ppm): 7.26-7.71 (1H, m), 7.75-7.82 (1H, m), 8.03 (1H, dd, J=1.2 Hz, J=8.4 Hz), 8.10 (1H, d, J=8.4 Hz), 8.38 (1H, s), 9.08 (1H, s) |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping