|
|
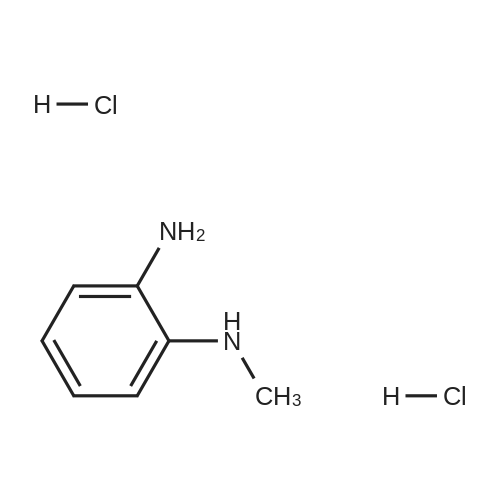
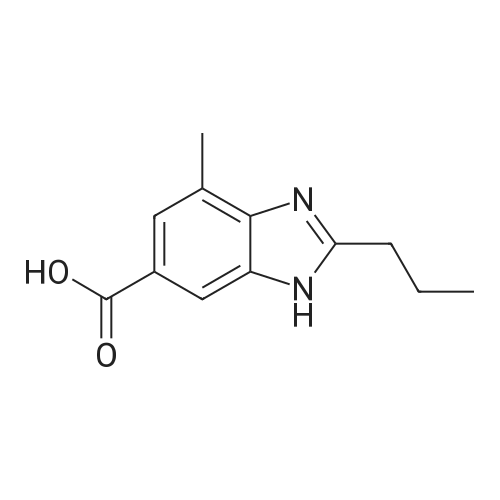
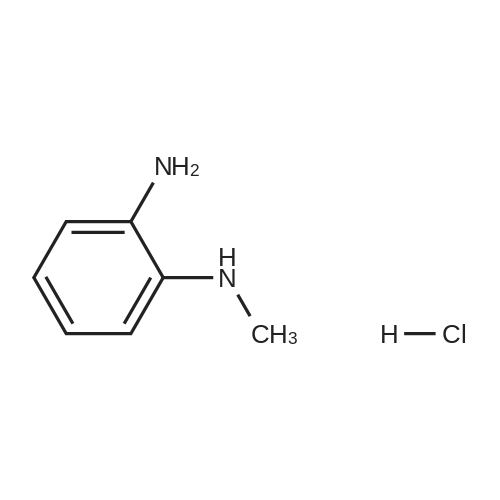
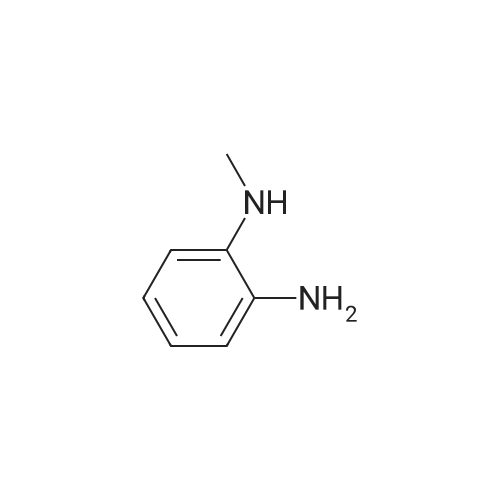
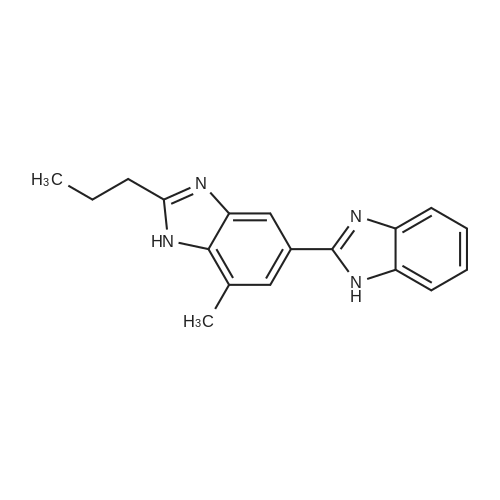
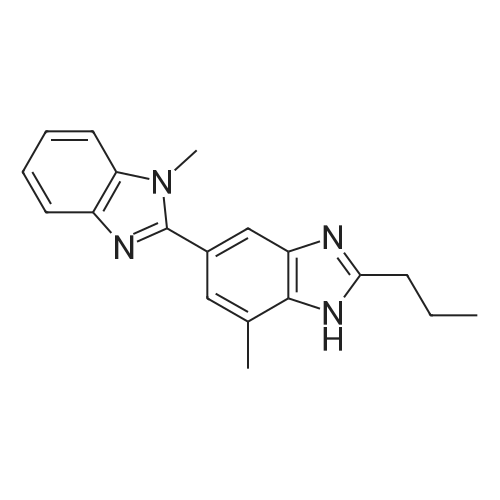
Stirring blade, a thermometer, three-neck flask 1L fitted with condenser, 4-methyl--2-n-propyl -1H- benzimidazole-6-carboxylic acid 50.0g (229mmol) and polyphosphoric acid added and stirred and 300 g, heated to around 70 C., the reaction solution obtained was stirred for 30 minutes, added in portions over a period N- methyl -O- phenylenediamine 45.0g of (368mmol) 2 hours It was.After adding the entire amount, stirring for 1 hour at around 70 C., and stirred for 5 h warmed to near yet 130. C..After confirming the disappearance of 4-methyl--2-n-propyl -1H- benzimidazole-6-carboxylic acid by HPLC, was cooled to about 70 C., while maintaining the water 600g The temperature of the reaction solution at 70 to 85 C. It was dropped little by little.After the total amount dropwise, 30 C. and cooled to near, pH of the reaction solution with aqueous ammonia was adjusted to be pH 8.3, after stirring for 1 hour warmed to around 50 C., the solid by vacuum filtration It is filtered off and washed the solid was filtered off with water 200g in the vicinity of 50 , to obtain a wet product of light brown crystals.Stirring blade, a thermometer, three-neck flask 1L fitted with condenser, was added and stirred the wet product of the resulting pale brown crystals and water 900 g, after stirring for 1 hour warmed to around 50 C. , vacuum filtered off the solids by filtration, wash the solid was filtered off with 100g of water around 50 C., the resulting wet product was dried for 15 hours under reduced pressure, a pale crude benzimidazole body as brown crystals to obtain a body 60.3g.The crude product of the benzimidazole body was analyzed by HPLC, the purity was 98.20%, the content of methyl benzimidazole body 0.14%, the content of ethyl benzimidazole body with 0.07% there were. stirring blade, a thermometer, three-necked flask 100mL fitted with a condenser, a mixture of a crude product 5.0g of methanol 12g and water 50g benzimidazole body obtained in Production Example 1 was added after stirring on, the addition of hydrochloric acid 2.1g containing hydrogen chloride 0.78 g (21.4 mmol), stirred for 10 minutes at about 25 C., the solid in the reaction solution was confirmed to be dissolved.Then, was added aqueous ammonia 1.1g containing ammonia 0.28 g (16.5 mmol), precipitation of white crystals was confirmed.After stirring for 1 hour at about 25 C., vacuum filtered through the solid was filtered off, the solid was filtered off with water 5g were washed twice and dried for 12 hours at 60 C. under reduced pressure and the resulting wet biomass, to obtain a benzimidazole body 4.2g (13.9mmol) as white crystals.Recovery rate, which is calculated based on the mass of the crude product of the benzimidazole body was 84.4%.As a result of the benzimidazole body obtained was analyzed by HPLC, the purity was 99.56%, the content of methyl benzimidazole body 0.06%, the content of ethyl benzimidazole body 0.02% met. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping