|
|
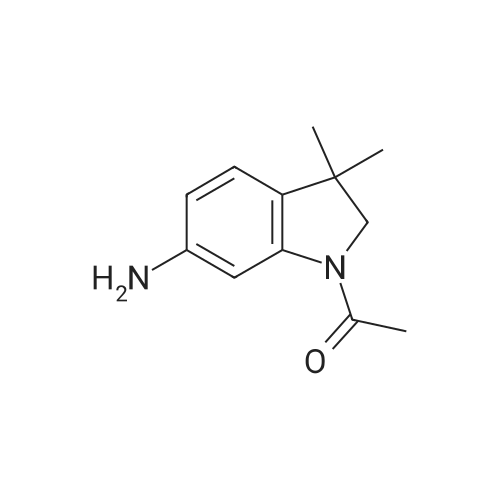
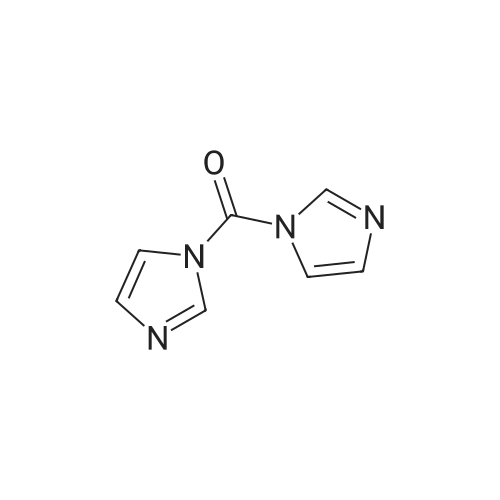
Stage g: 3-(1-Acetyl-3,3-dimethyl-2,3-dihydro-1H-indol-6-yl)-5,5-dimethylimidazolidine-2,4-dione A solution of 6.6 g of 1,1'-carbonylbis(1H-imidazole) and 460 mg of 1H-imidazole in 50 mL of tetrahydrofuran is stirred under argon and cooled in an ice bath at 0 C. To this solution is added a suspension of 6.9 g of <strong>[453562-71-5]1-acetyl-3,3-dimethylindolin-6-amine</strong> obtained in stage f) below in 50 mL of tetrahydrofuran. After stirring for one hour, 9.5 mL of triethylamine and 5.2 g of methyl 2-methylalaninate hydrochloride are added and the mixture is stirred for two hours at room temperature and then refluxed for 17 hours. After cooling to room temperature, the mixture is diluted with 800 mL of water and the precipitate formed is filtered off, washed with four times 25 mL of water and with three times 15 mL of diethyl ether and then dried to give 8 g of 3-(1-acetyl-3,3-dimethyl-2,3-dihydro-1H-indol-6-yl)-5,5-dimethylimidazolidine-2,4-dione in the form of a beige-coloured solid, the characteristics of which are as follows: LCMS: RT=3.19 min; m/z=316 [M+H]+; m/z=314 [M-H]- |
|
|
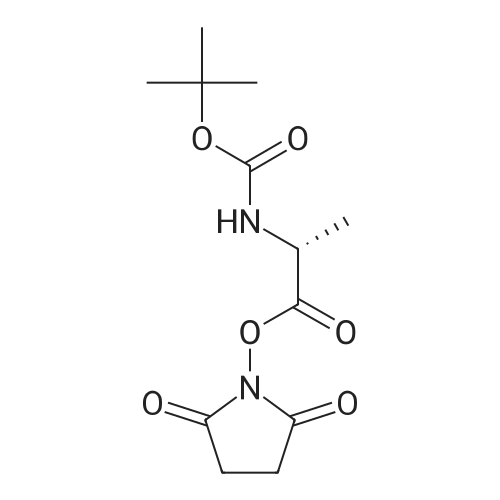
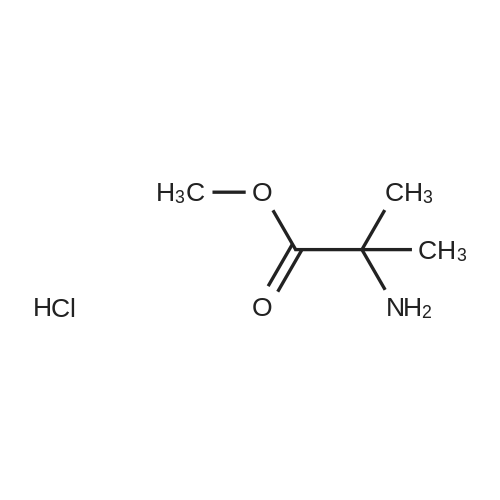
Steps A ? and B:5,17 mmol ' of 1,1' -Carbonyldimidazole and 0,86- -mmo. ofimidazole are dissolved in 10 ml THF and cooled'to 0C. Asolution of 'the aromatic amine . (4,31 mmol ) in a .suitable'amount of THF' '(5 to 10 ml) is added over .15 mini..The.reaction mixture "is allowed to 'reach RT and stirred, f.oranother 2h. Then 4,3 'mmol of Net3 and 4,3 mmol .of ..2-'amino-2-methyl-propionic = acid methyl. ester ' .acid?hydrochlorid are added and ' the resulting mixture -,isstirred''until completion of the reaction. After theevaporation of the solvent ' the crude product is pure,enough ? for ' the next step. Example 293- (l-Acetyl-3, 3-dimethyl-2, 3-dihydro-lH-indol-6-yl) -5, 5-? dimethyl-1- [2- (pyrazin-2- ylamino) -pyridin-4-ylmethyl] -? imi-dazoridine-2, 4-dione? Start-ing /from 1- (6^Amino-3, 3'-dimethyl-2, 3-dihydro-indol-1-yl) -ethanohe in step ? A and' using steps,, B, C, D .arid* Ewith 2-aminopyrazine?M+H+ measured =? 50-0.24LC/MS retention time [min] =1.3.3- Example 7la ' .- . . , .3-(l-Acetyl-3,3-dimethyl-2,3-dihydro-lH-indol-6-yl)-5,5-dimethyl-imidazolidine-2,4- dione To a ' solution." of -838 mg d'i-imidazol-1-yl-methanone..and 58. mg imidazole in 10 ml ? tetrahydrofuran a solution of 880 mg ? 1-(6-amino-3f3-dimethyl-2,3-dihydro-indol-l-yl)-ethanone in 5 ml tetrahydrofuran was slowly added at 0C. After stirring at 0C for 90 minutes. 0.60 ml triethylamine and 661 mg 2-amino-2-methyl-propionic acid methyl- ester hydrochloride were added and the reaction mixture was allowed to warirv up to room temperature. After, 2 hours stirring at room temperature the solution was ?heated for 6 hours at - 70C.' After cooling to ,room temperature'the solvent of the mixture was removed under reduced pressure and ? the residue was purified by flash chromatography on silica gel with a n-heptane/ ethylacetate gradient. The fractions -containing the product were combined .and evaporated to yield a white solid.Yield: 920 mg'M+H-f- measured = 3161H-NMR (400 MHz, DMSO/TMS) : d = 8.50 (s, 1H) ; 7.93 (s, 1H);'7.33 (d, 1H) ; 6.97. - (dd, ? 1H) ; 3.90 (s, 2H) ; 2.17 (s, 3H); 1.50 (s, 6H); 1.33 (s, 6H) |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping