|
|
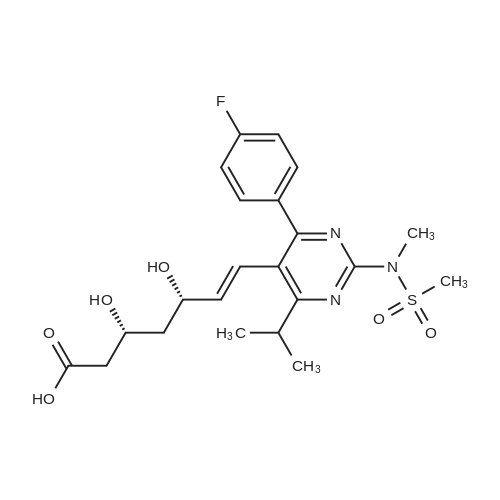
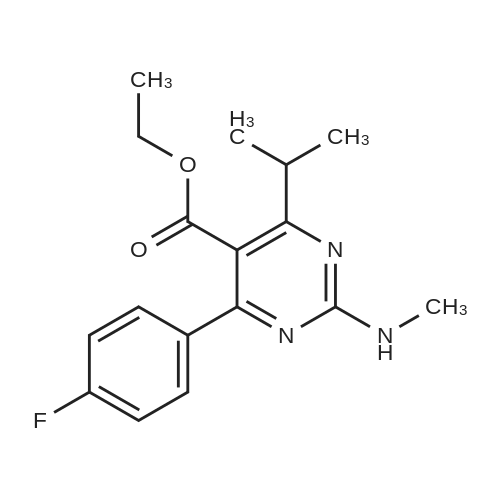
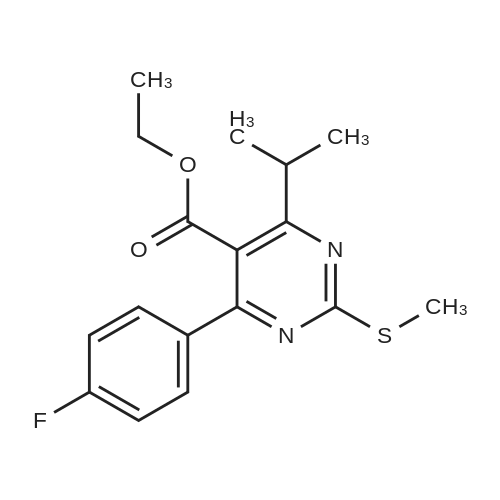
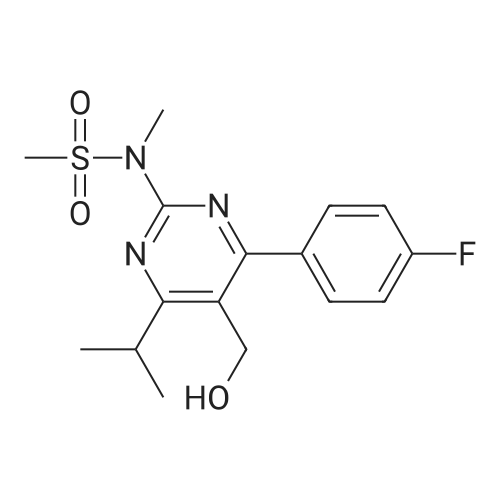
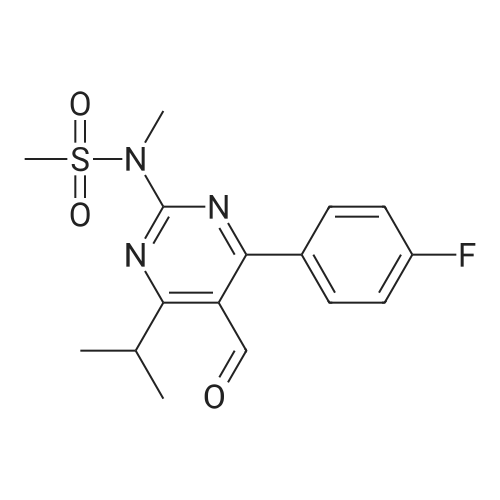
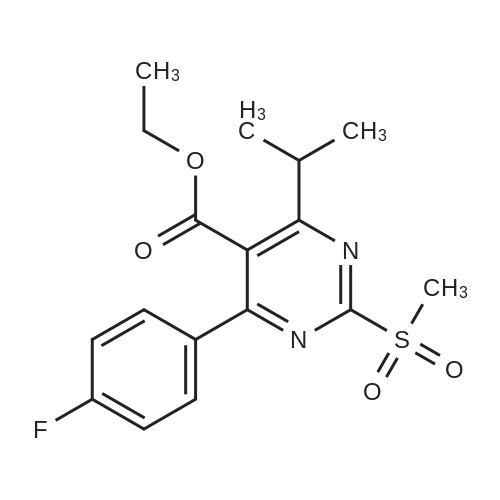
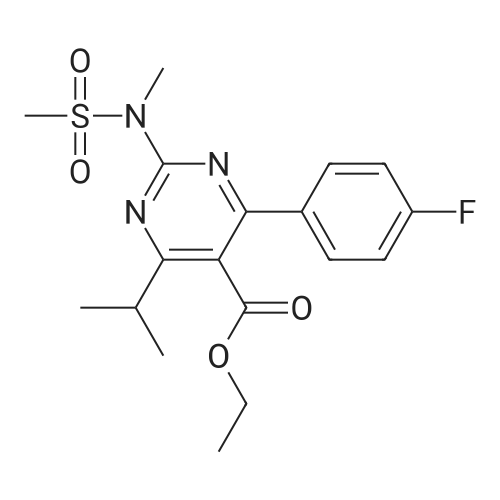
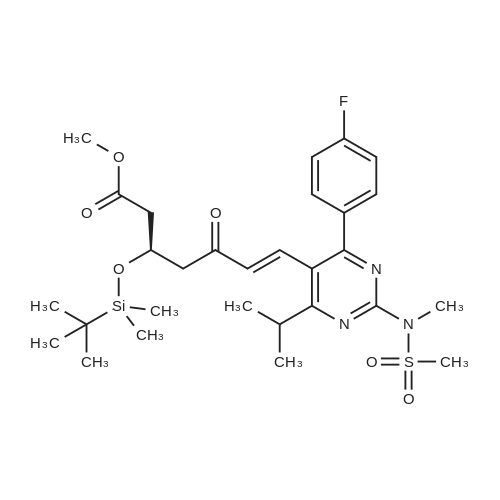
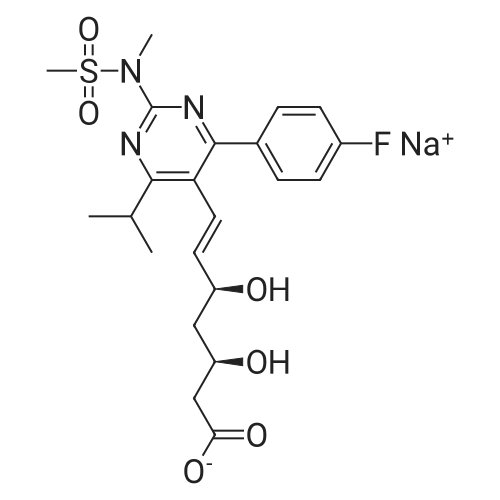
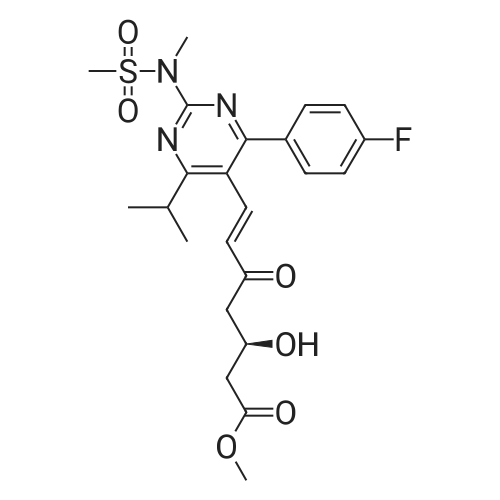
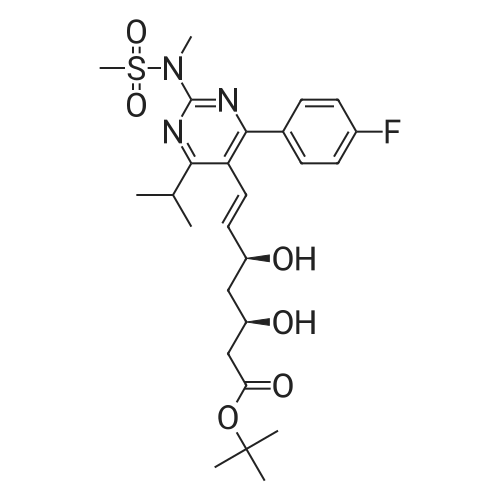
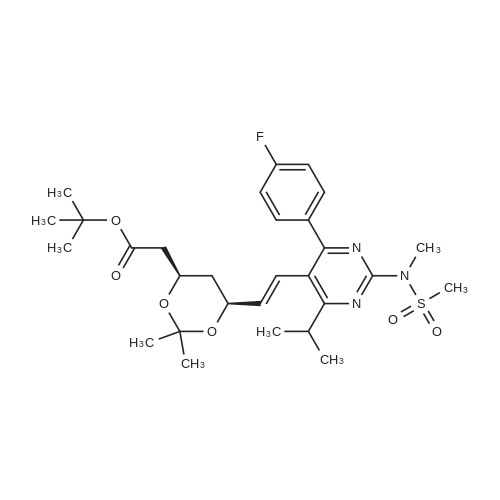
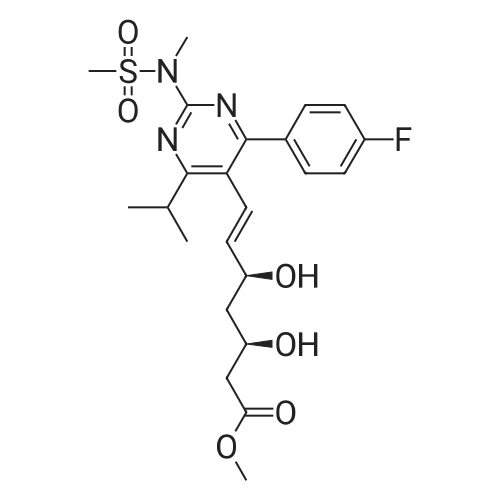
Tentative Example 3 Preparation of 2-((4/?,6S)-6-((E)-2-(4-(4-fluorophenyl)-6-isopropmethylmethylsulfonamido)pyrimidin-5-yl)vinyl)-2,2-dimet^^ 4-methylpentan-2-yl ester from N-(4-(4-fluorophenyl)-5-formyl-6- isopropylpyrimidin-2-yl)-N-methylmethanesulfonamide (D) and 2-((4 ?,6S)-6- ((benzo[d]thiazol-2-ylsulfonyl)methyl)-2,2-dimethyl-1 ,3-dioxan-4-yl)acetate 4- methylpentan-2-yl esterThe 4-methylpentan-2-yl ester of compound (5a) with is a radical of formula (D), R3 and R4 are both methyl and R5 is 4-methylpentan-2-yl can be prepared according to the same procedures as outlined in Example 3 starting from 2-((4f?,6S)-6-((benzo[d]thiazol- 2-ylsulfonyl)methyl)-2,2-dimethyl-1 ,3-dioxan-4-yl)acetate 4-methylpentan-2-yl ester instead of the corresponding sec-butyl ester using the same molar amount.For reference purposes 2-((4f?,6S)-6-((E)-2-(4-(4-fluorophenyl)-6-isopropyl-2-(N- methylmethylsulfonamido)pyrimidin-5-yl)vinyl)-2,2-dimethyl-1 ,3-dioxan-4-yl)acetate 4- methylpentan-2-yl ester was prepared from <strong>[147118-40-9]rosuvastatin methyl ester</strong> (EP 521471). Thus, <strong>[147118-40-9]rosuvastatin methyl ester</strong> (30 g, 61 mmol) was added to 200 mL of acetonitrile and 2N aqueous NaOH was added until the pH was stable at 12.5. The reaction mixture was stirred for 2 h at 20°C. Then the pH was lowered to 5.0 with 2N aqueous HCI. To the reaction mixture was added 200 mL of ethyl acetate and the organic phase was separated and washed 2 times with 100 mL of water. The ethyl acetate phase was dried over Na2S04, filtered and evaporated to a sirup (" 30 g of rosuvastatin acid). Part of this sirup (20 g) was dissolved in toluene and heated to reflux under azeotropic water removal for 4 h. The reaction mixture was cooled to 20°C and stirred for 18h. The precipitated solid was filtered, washed with toluene (2 x 10 mL) and dried to give 16.1 g (35 m mol) of N-(4-(4-fluorophenyl)-5-((E)-2-((2S,4 )-4-hydroxy-6-oxotetrahydro-2H- pyran-2-yl)vinyl)-6-isopropylpyrimidin-2-yl)-N-methylmethanesulfonamide as a white solid. 1 H NMR (300 MHz, CDCI3) delta 7.62 (dd, 2H), 7.1 1 (dd, 2H), 6.72 (dd, 1 H), 5.48 (dd, 1 H), 5.28-5.20 (m, 1 H), 4.38-4.30 (m, 1 H), 3.58 (s, 3H), 3.52 (s, 3H), 3.38-3.30 (m, 1 H), 2.80-2.60 (m, 2H), 2.10-2.00 (m, 1 H), 1 .95-1 .85 (m, 1 H), 1 .73-1.68 (m, 1 H), 1 .28 (d, 3H), 1.26 (d, 3H). Of this compound, 2.3 g (5.0 mmol) was added to 25 mL of 2-(4-methyl)-pentanol. Then 2 drops of methanesulphonic acid were added and the reaction mixture was heated to 60°C and stirred for 1 h. The reaction mixture was cooled to 20°C and stirred for 18h. Next 2,2-dimethoxypropane (0.78 g, 7.5 mol) was added and the mixture was stirred for 2h. The reaction mixture was quenched with 20 mL of saturated aqueous NaHC03 followed by addition of 25 mL of ethyl acetate. The organic phase was separated and washed 2 times with 20 mL of saturated aqueous NaHC03 The organic phase was evaporated and the residue slowly solidified to give the title compound as a solid (2.9 g, 96percent yield). 1 H NMR (300 MHz, CDCI3) delta 7.58 (dd, 2H), 7.01 (t, 2H), 6.46 (dd, 1 H), 5.42 (dd, 1 H), 4.87 (m, 1 H), 4.43 - 4.20 (m, 2H), 3.50 (s, 3H), 3.45 (s, 3H), 3.35 - 3.25 (m, 1 H), 2.38 (ddd, 2H), 1.59 - 1.43 (m, 4H), 1.40 (s, 3H), 1.35 (s, 3H), 1.20, (dd, 4 H), 1.14 (d, 6H), 0.83 (t, 6H). |
|
|
For reference purposes 2-((4R,6S)-6-((E)-2-(4-(4-fluorophenyl)-6-isopropyl-2-(N-methylmethylsulfonamido)pyrimidin-5-yl)vinyl)-2,2-dimethyl-1,3-dioxan-4-yl)acetate 4-methylpentan-2-yl ester was prepared from <strong>[147118-40-9]rosuvastatin methyl ester</strong> (EP 521471). Thus, <strong>[147118-40-9]rosuvastatin methyl ester</strong> (30 g, 61 mmol) was added to 200 mL of acetonitrile and 2N aqueous NaOH was added until the pH was stable at 12.5. The reaction mixture was stirred for 2 h at 20° C. Then the pH was lowered to 5.0 with 2N aqueous HCl. To the reaction mixture was added 200 mL of ethyl acetate and the organic phase was separated and washed 2 times with 100 mL of water. The ethyl acetate phase was dried over Na2SO4, filtered and evaporated to a sirup (?30 g of rosuvastatin acid). Part of this sirup (20 g) was dissolved in toluene and heated to reflux under azeotropic water removal for 4 h. The reaction mixture was cooled to 20° C. and stirred for 18 h. The precipitated solid was filtered, washed with toluene (2×10 mL) and dried to give 16.1 g (35 m mol) of N-(4-(4-fluorophenyl)-5-((E)-2-((2S,4R)-4-hydroxy-6-oxotetrahydro-2H-pyran-2-yl)vinyl)-6-isopropylpyrimidin-2-yl)-N-methylmethanesulfonamide as a white solid. 1H NMR (300 MHz, CDCl3) delta 7.62 (dd, 2H), 7.11 (dd, 2H), 6.72 (dd, 1H), 5.48 (dd, 1H), 5.28-5.20 (m, 1H), 4.38-4.30 (m, 1H), 3.58 (s, 3H), 3.52 (s, 3H), 3.38-3.30 (m, 1H), 2.80-2.60 (m, 2H), 2.10-2.00 (m, 1H), 1.95-1.85 (m, 1H), 1.73-1.68 (m, 1H), 1.28 (d, 3H), 1.26 (d, 3H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping