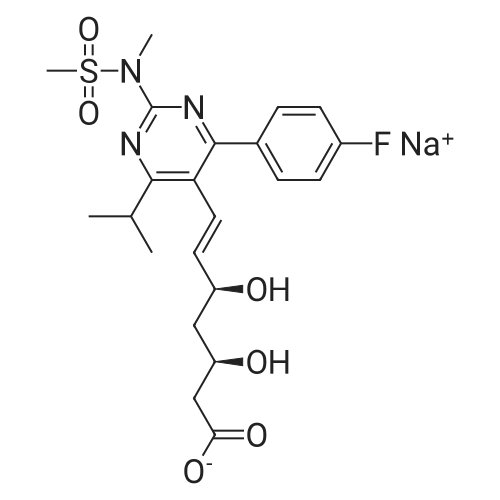
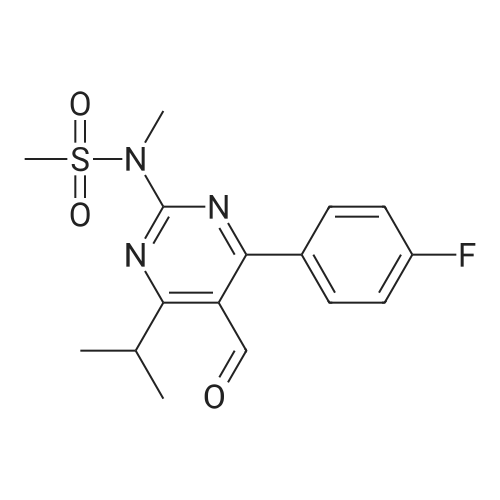
| 92% |
With N-butylammonium bromide; In toluene; acetonitrile; at 75℃; for 12h; |
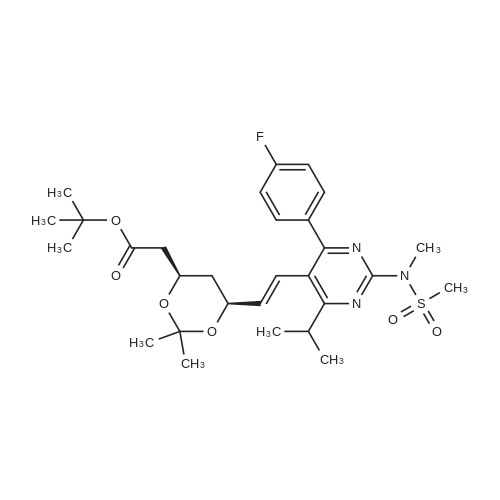
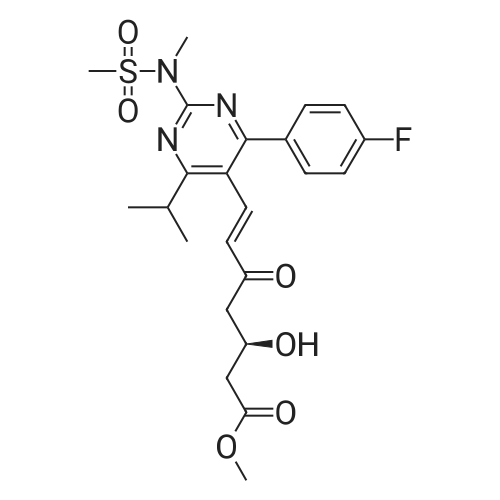
A mixture of 400 g of acetonitrile and 200 g of toluene was added to a 2 L reaction flask, and 100 g of the main chain Z8 and 160 g of the side chain J6 were charged with stirring, 2 g of 4-butylammonium bromide was added, and the mixture was heated to 75 C Reflux for 12 hours, the end of the reaction, 40 C, -0.08MPa under the conditions of concentration under reduced pressure to get the crude oil that is H1. b. Condensation product purification: Add 1400 ml of n-hexane and 1200 ml of petroleum ether mixture to the oil, heat to reflux, cool the solution to 0 C, stir for solid for 6 hours, continue to warm to 20 C, stir for 30 min , The filter was removed by filtration, the filter cake was washed with petroleum ether, the filtrate and the washing liquid were combined, and the filtrate was distilled under reduced pressure at 40 C and -0.08 MPa to remove the organic solvent to obtain the product as H1. The detection purity was 91.4% and the yield was 92% |
| 71.3% |
In acetonitrile; for 14h;Heating / reflux; |
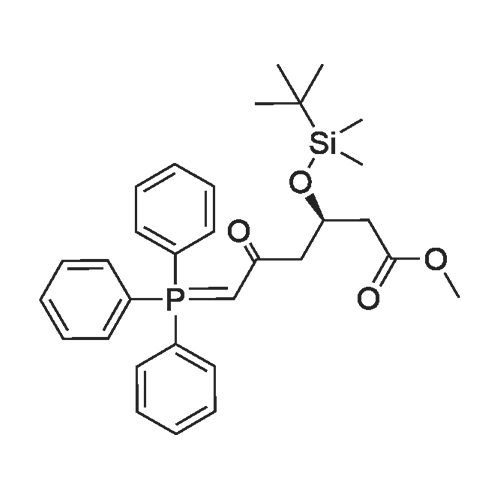
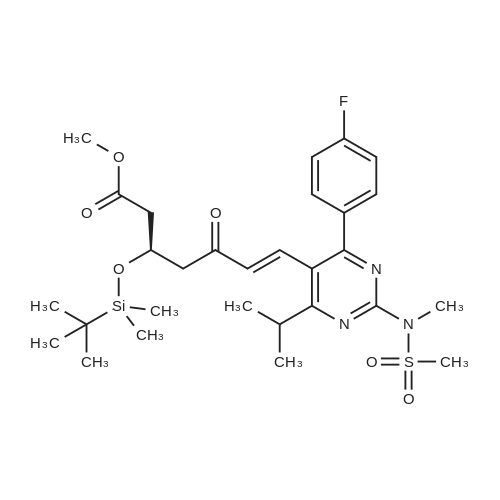
(3); A solution of 190 mg of FPP-OH (compound (i)), 450 mg of methyl (3R)-3-(tert-butyldimethylsilyloxy)-5-oxo-6-triphenylpliosplioranylidenehexanate5 (prepared in step 2 above) and 5 mL of acetonitrile is refluxed under heating for 14 hours and evaporated under reduced pressure to distill off acetonitrile. The resulting residue is subjected to column chromatography on silica gel eluting with methylene chloride to give 233 mg (Yield: 71.3%) of methyl 7-[4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N-methylsulfonylamino)pyrimidin-5-yl)-(3R)-3-(tert-butyldimethylsilyloxy)-5-oxo-(E)-6-heptenate (compound ii) as a syrup. |
|
|
A 250 ml flask, protected from light and provided with N2 flow was charged with compound-14 (4.38 g, 12.5 mmol), compound 19M (10 g, 18.7 mmol), and extra dry toluene (100 ml). The reaction mixture was heated to about 1000C for 15 hrs. After the completion of the reaction, anhydrous MgCl2 (4.8 g, 2.7 eq.) was added to the reaction mixture and the reaction mixture was heated for 2 hours at about 1000C. The reaction mixture was cooled to O0C over a period of about 2 hours, filtered, and washed with 45ml of toluene, yielding 12.73g of a viscous oil. |
|
In toluene; at 110℃; for 24h;Product distribution / selectivity; |
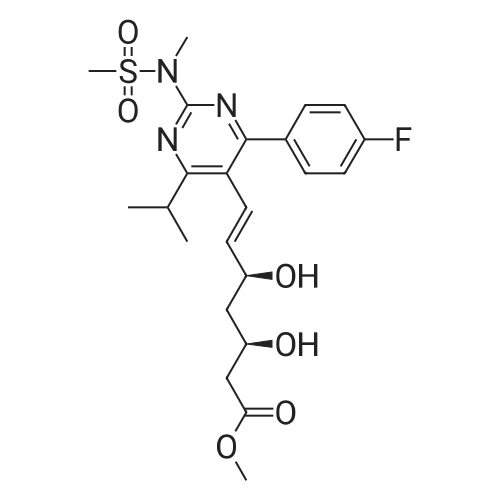
EXAMPLE 1 : PREPARATION OF METHYL-7- [4-(4-FLUOROPHENYL)-6-ISO- PROPYL-2- (N-METHYL-N-METHYLSULPHONYLAMINO) PYRIMIDIN-5-YL] - (3R)-3-HYDROXY-5-OXO- (E)-6-HEPTENATE (FORMULA V):; Methyl (3R)-3-(tert-butyl dimethyl silyloxy)-5-oxo-6-triphenyl- phosphoranyllidene hexanoate (10 g), N-[4-(4-Fluoro-phenyl)-5-formyl-6-isopropyl- pyrimidin-2-yl]-N-methyl-methanesulfonamide (6.9 g), toluene (100 ml) were taken into a round bottom flask and heated to about 110 0C. The reaction mass was maintained at the same temperature for about 24 hours. Reaction completion was checked using thin layer chromatography. After the reaction was completed, the reaction mass was distilled at a temperature of about 45 0C under low pressure. To the residue obtained, a 1 : 9 mixture of ethyl acetate and n-hexane (100 ml) was added and stirred at about 26 0C for about 1 hour. The mixture was then cooled to about 10 0C and maintained under stirring for another 1 hour. The separated solid was filtered, and the filtrate was distilled off completely under high vacuum at about 45 0C. To the residue obtained, acetonitrile (130 ml) was added at about 26 0C, and then further cooled to about 5 0C. The mixture was maintained at 5 0C for about 15 minutes and then a solution of 48% aqueous hydrogen fluoride in acetonitrile (13 ml) was added to it and maintained at the same temperature for about 1 hour. The reaction mass was then allowed to heat to 26 0C and maintained for about 1 hour. Reaction completion was checked using thin layer chromatography. After the reaction was completed, saturated sodium bicarbonate solution (140 ml) was added to the reaction mass until pH was adjusted to 7.0. Dichloromethane (300 ml) was added to the above reaction mass and stirred for about 10 minutes. The organic layer was separated and the aqueous layer was extracted into dichloromethane (100 ml). The combined dichloromethane layer was distilled off at about 39 0C under high vacuum to give 11.2 g of the title compound. |
|
In acetonitrile; at 80℃; for 24h;Product distribution / selectivity; |
EXAMPLE 2: PREPARATION OF METHYL-7- [4-(4-FLUOROPHENYL)-6-ISO- PROPYL-2- (N-METHYL-N-METHYLSULPHONYLAMINO) PYRIMIDIN-5-YL] - (3R)-3-HYDROXY-5-OXO- (E)-6-HEPTENATE (FORMULA V):; Methyl (3R)-3-(tert-butyl dimethyl silyloxy)-5-oxo-6-triphenyl- phosphoranyllidene hexanate (100 g), N-[4-(4-fluoro-phenyl)-5-formyl-6-isopropyl- pyrimidin-2-yl]-N-methyl-methanesulfonamide (69 g), acetonitrile (1000 ml) were taken into a round bottom flask and heated to about 80 0C. The reaction mass was maintained at the same temperature for about 24 hours. Reaction completion was checked using thin layer chromatography. After the reaction was completed, the reaction mass was distilled at a temperature of about 48 0C under reduced pressure. To the residue obtained (175 g), acetonitrile (875 ml) was added and stirred at about 26 0C for about 1 hour. The mixture was then cooled to about 5 0C and a solution of 48% aqueous hydrogen fluoride (36 ml) was added to it. After the addition was completed, the reaction mass was then allowed to heat to about 26 0C and maintained for about 1 hour. After the reaction was completed, saturated sodium bicarbonate solution (610 ml) was added to the reaction mass until the pH was adjusted to 7.0. Dichloromethane (1750 ml) was added to the above reaction mass and stirred for about 10 minutes. The organic layer was separated and the aqueous layer was extracted into dichloromethane (855 ml). The combined dichloromethane layer was washed with saturated sodium chloride solution (885 ml) and then distilled off at about 39 0C under high vacuum to give 145 g of the title compound as a residue. To 8.0 g of the residue obtained, a 9:1 mixture of n- hexane in ethyl acetate (150 ml) was added and stirred at about 25 0C for about 1 hour. The separated solid was filtered and washed with a 9: 1 mixture of n-hexane in ethyl acetate (125 ml) to yield 5.5 g of the title compound. |
|
In acetonitrile; at 84℃; for 12h; |
The synthetic route is shown in flow diagram 1: The detailed preparation process is as follows: 165mL acetonitrile, 30.7g (R)-3-[[(1,1-dimethyl ethyl)dimethyl silyl]oxo]-5-oxo-6-(triphenyl phosphoranylidene)-methyl hexanoate and 20.2g N-[4-(4-flurophenyl)-5-formyl-6-(1-methylethyl)-2-pyrimidyl]-N-methyl-methanesulfonamide were added to a 200mL reaction flask. Subsequently, the mixture was heated to 84C and refluxed until TLC indicated that the content of N-[4-(4-flurophenyl)-5-formyl-6-(1-methylethyl)-2-pyrimidyl]-N-methyl-methanesulfonamide in the reaction solution did not change any more. This reaction took around 12 hours, then the reaction mixture was cooled and vacuum distilled at 50C to remove acetonitrile. In this way oily product was obtained. The oily product was immediately transferred to a 500mL reaction flask, subsequently, 345mL cyclohexane was added, the mixture was heated and refluxed for 20 minutes, and then the reaction mixture was cooled to 5C and stirred for 5 hours until solid precipitated. The obtained product was filtered to remove the solid, the filter cake was washed twice with cyclohexane (40mL each time), and then the washing solution was combined with filtrate. The obtained filtrate was vacuum distilled to remove the organic solvent cyclohexane at 45C. As a result, 46g oily (3R,6E)-3-[[(1,1-dimethyl ethyl) dimethyl silyl]-oxo]-7-[4-(4-flurophenyl)-6-(1-methyl ethyl)-2-[methyl (methyl sulfonyl) amino]-5-pyrimidyl]-5-oxo-6-methyl heptenoate was obtained. |
|
In cyclohexane; at 25 - 85℃; |
Example 1Preparation of methyl 7-[4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N- methylsulfonyIamino)pyrimidin-5-yI]-(3R)-3-(ter^butyldimethylsilyloxy)-5-oxo-( - 6-heptenate (IV)4-(4-Fluorophenyl)-6-isopropyl-2-(N-methyl-N-methylsulfonylamino)-5- pyrimidinecarbaldehyde (lOOg) was taken in cyclohexane (500 ml) and to this methyl (3 ?)-3-(tert-butyldimethylsilyloxy)-5-oxo-6-triphenylphosphoranylidene hexanoate (160g) was added at 25-30C. The reaction mixture was refluxed under azeotropic condition at 80-85 C for 48-60 hours under stirring. After completion of reaction, the reaction mixture was cooled and stirred for 2.0 hours. The reaction mass was filtered and washed with cyclohexane. The organic layer was concentrated under reduced pressure to obtain the title compound as thick oily mass. |
|
In acetonitrile; for 30h;Reflux; |
A 500 ml four-necked flask was charged with 4- (4-fluorophenyl) -6-isopropyl-2 (N-methyl-N-methanesulfonamido) pyrimidin- 5-yl] -carbaldehyde (Z9, 10 g, 28.5 mmol),(3R) -3-tert-butyldimethylsilyloxy-5- carbonyl-6- (triphenylphosphinoethenyl) -hexanoic acid methyl ester (J6, 16.76 g, 31.3 mmol) and acetonitrile ,Reflux reaction for about 30h, HPLC monitoring, until the reaction is complete, concentrated to dryness under reduced pressure, were added cyclohexane 60ml spin-dry strip twice,Then 200 ml of cyclohexane was added and the reaction was refluxed for 1 hour. The mixture was cooled to 10-15 C for 2 hours and filtered. The filtrate was concentrated to dryness under reduced pressure and directly used for the next reaction. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping