|
With N,N-dimethyl-aniline; trichlorophosphate; at 130℃; for 2h; |
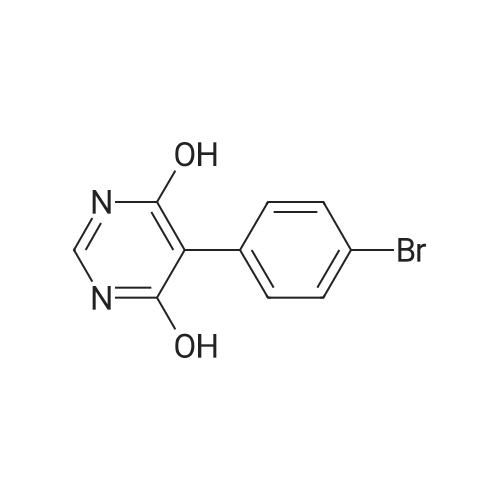
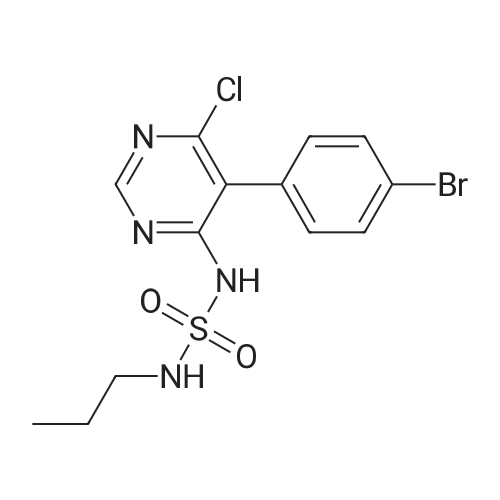
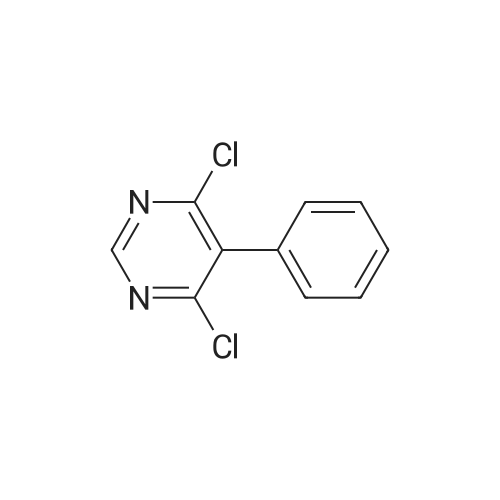
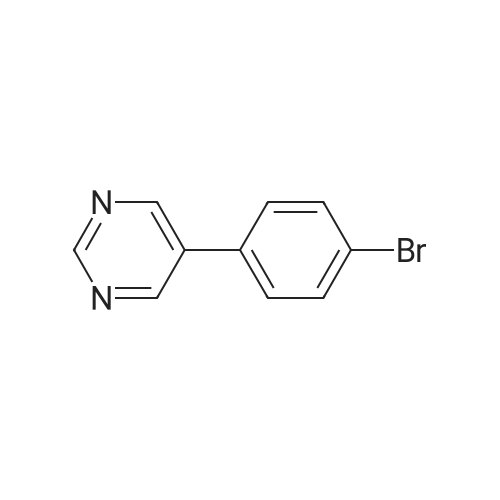
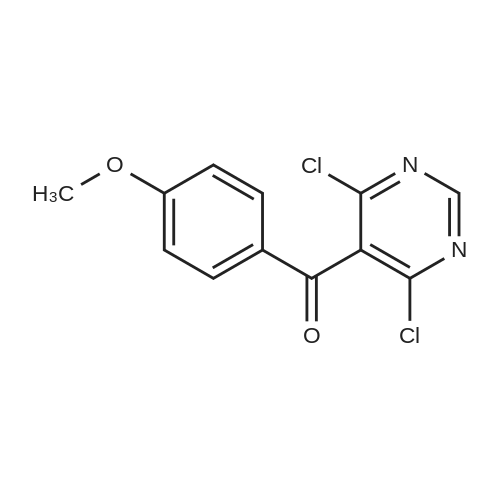
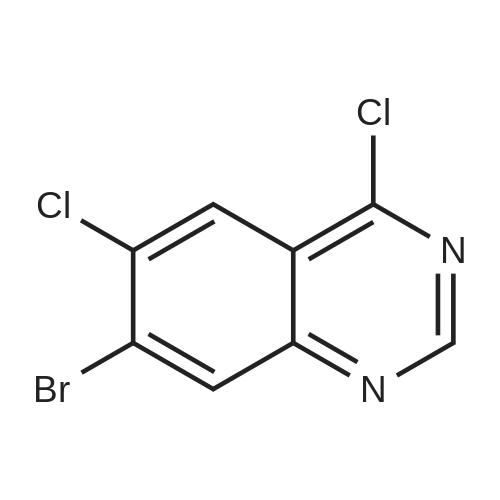
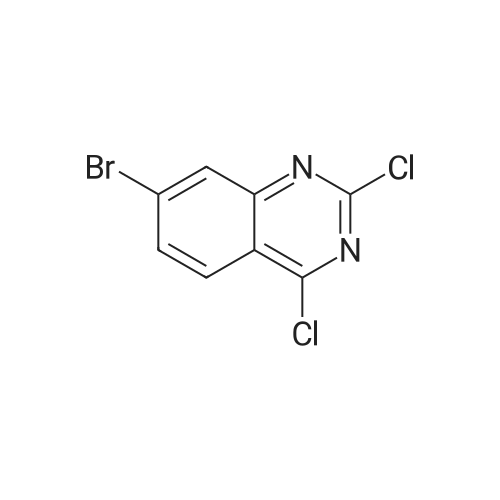
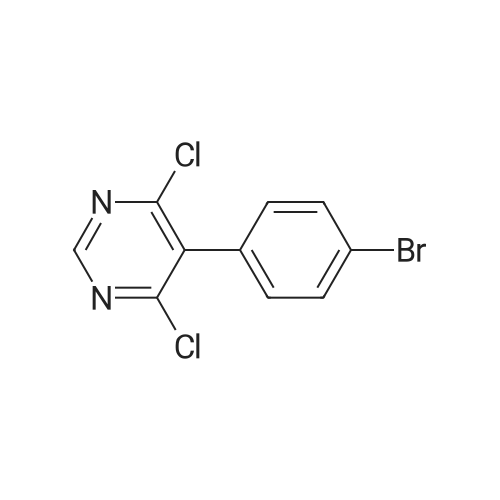
To a suspension of 5-(4-bromophenyl)-pyrimidine-4,6-diol (9.9O g) in POCl3 (13O mL) was carefully added JV,lambda/-dimethylaniline (13.5 mL). The mixture is heated to 1300C for 2 h. The dark brown solution is concentrated in vacuo and the residue was poured into ice/water. The suspension is diluted with 2 N HCl and water and stirred for 20 min. The precipitate that formed is collected and washed with water. The solid material is dissolved in EA, washed with 1 N aq. HCl and brine. The org. phase is dried over MgSO4 and evaporated. The material is further purified by column chromatography on silica gel eluting with Hex:EA 95:5 to 1 :1 followed by crystallisation from Hex/EA at -200C to give 4,6-dichloro-5-(4-bromophenyl)-pyrimidine as pale yellow crystals (8.3 g). 1H-NMR(D6-DMSO): delta 7.39-7.44 (m, 2H), 1.12-1.16 (m, 2H), 8.94 (s, IH). |
|
With N,N-dimethyl-aniline; trichlorophosphate; at 130℃; for 2h; |
d) To a suspension of 5-(4-bromophenyl)-pyrimidine-4,6-diol (9.90 g) in POCI3 (130 ml_) is carefully added N, N-dimethylaniline (13.5 ml_). The mixture is heated to 1300C for 2 h. The dark brown solution is evaporated and the residue is poured into ice/water. The suspension is diluted with 2 N HCI and water and stirred for 20 min. The precipitate is collected and washed with water. The solid material is dissolved in EA, washed with 1 N aq. HCI and brine. The organic phase is dried over MgSO4 and evaporated. The material is further purified by column chromatography on silica gel eluting with hexane:EA 95:5 to 1 :1 followed by crystallisation from hexane/EA at -200C to give 4,6-dichloro-5-(4-bromophenyl)- pyrimidine (8.3 g) as pale yellow crystals. 1H-NMR(D6-DMSO): 7.39-7.44(m, 2H)1 7.72-7.76(m, 2H), 8.94(s, 1 H). |
|
|
To a suspension of 5-(4-bromophenyl)-pyrimidine-4,6-diol (9.90 g) in POCl3 (130 mL) was carefully added N,N-dimethylaniline (13.5 mL). The mixture is heated to 130 C. for 2 h. The dark brown solution is concentrated in vacuo and the residue was poured into ice/water. The suspension is diluted with 2 N HCl and water and stirred for 20 min. The precipitate that formed is collected and washed with water. The solid material is dissolved in EA, washed with 1 N aq. HCl and brine. The org. phase is dried over MgSO4 and evaporated. The material is further purified by column chromatography on silica gel eluting with Hex:EA 95:5 to 1:1 followed by crystallisation from Hex/EA at -20 C. to give 4,6-dichloro-5-(4-bromophenyl)-pyrimidine as pale yellow crystals (8.3 g).1H-NMR(D6-DMSO): delta 7.39-7.44 (m, 2H), 7.72-7.76 (m, 2H), 8.94 (s, 1H).LC-MS: tR=1.02 min. |
| 100 g |
With triethylamine; trichlorophosphate; In acetonitrile; at 25 - 85℃; for 5h;Inert atmosphere; |
Triethyl amine (130 mL) was added to a mixture of 5-(4-bromophenyl)-pyrimidine-4,6-diol (100 g), phosphoryl chloride (400 mL) and acetonitrile (600 mL) at 25-35C under nitrogen atmosphere for a period of 60 mins. The reaction mass was heated to 80-85C and stirred for 4 hrs. The reaction mass was distilled off under vacuum at below 75C followed by stripped off with acetonitrile. The reaction mass was cooled to 10-15C, water (500 mL) was added and stirred for 30 mins. Aqueous hydrochloric acid (2N, 500 ml) was added to the reaction mass at 10-15C and stirred for 30 mins. The solid obtained was filtered, washed with water and dried under vacuum at 40-45C for 6 hrs to get the title compound. Yield: 100 g; purity by HPLC: 99.0% |
|
With N,N-dimethyl-aniline; trichlorophosphate; at 70℃; for 4h; |
Add 5-(4-bromophenyl)-4,6-dihydroxypyrimidine to a washed, dry round bottom flask.70 parts of phosphorus oxychloride (new steam),Then, 1 part of N,N-dimethylaniline was added dropwise thereto.Slowly turn on the magnetic stirrer and stir at room temperature for 15 minutes.Warmed to 70 C in 1 h,The reaction was carried out at this temperature for 3 h.After the reaction was terminated, it was naturally cooled to 55 C, and excess phosphorus oxychloride was distilled off under reduced pressure.Add the remaining mixture to the ice water, the temperature is controlled below 10 C, the natural yellow solid will be precipitated, and the pH of the solution will be adjusted.To be weakly acidic, it was washed twice with ice water and then with dichloroethane three times. After drying the wet product, a pale yellow product can be obtained (5-(4-Bromophenyl)-4,6-dichloropyrimidine). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping