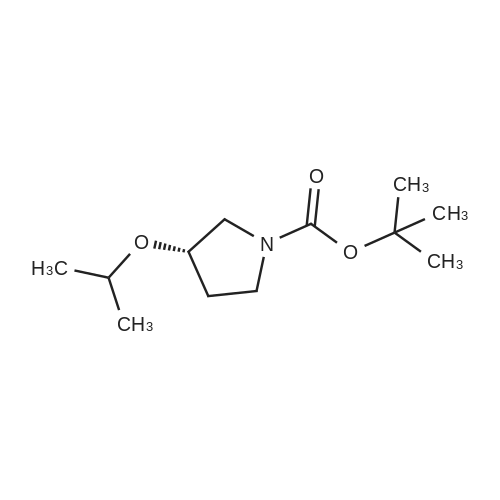
Alternatived Products of [ 146257-03-6 ]
Product Details of [ 146257-03-6 ]
| CAS No. : | 146257-03-6 |
MDL No. : | MFCD08752479 |
| Formula : |
C10H19NO3
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
201.26
|
Pubchem ID : | - |
| Synonyms : |
|
Safety of [ 146257-03-6 ]
Application In Synthesis of [ 146257-03-6 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 146257-03-6 ]
- 1
-
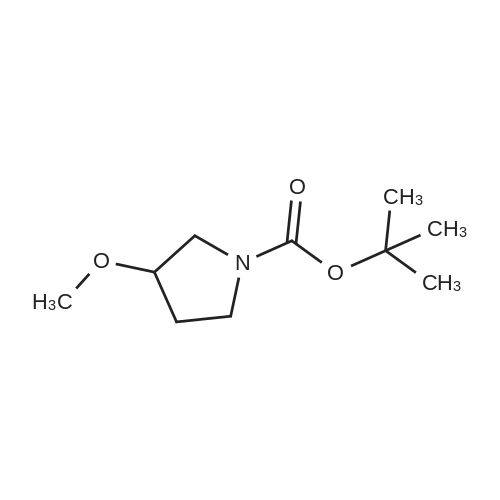 [ 146257-03-6 ]
[ 146257-03-6 ]

-
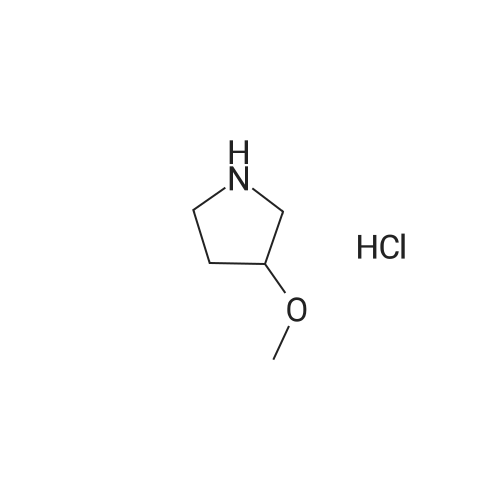 [ 136725-50-3 ]
[ 136725-50-3 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With hydrogenchloride; water; In tetrahydrofuran; for 1h; |
Procedure 32 Procedure 32 provides a method for the preparation of 3-alkoxypyrrolidines from N-Boc-3-hydroxypyrrolidine. 1-Boc-3-hydroxypyrrolidine (16.1 mmol) was added in portions to a suspension of sodium hydride (22.0 mmol) in tetrahydrofuran (40 mL) at 0 C. The reaction mixture was diluted with tetrahydrofuran (60 mL) and allowed to warm to rt. Methyl iodide (21.0 mmol) was added to the cloudy suspension after 1 h and the reaction mixture was maintained at rt for 6 h. The reaction mixture was concentrated and redissolved in ethyl acetate (100 mL). The extract was washed with saturated ammonium chloride (20 mL), water (20 mL), and brine (20 mL) and was dried (sodium sulfate). The residue was purified by chromatography (1/4 ethyl acetate/hexane) to give the ether. The N-Boc product was dissolved in tetrahydrofuran (30 mL) and 6 N hydrochloric acid (20 mL) was added. The resultant mixture was stirred for 1 h and was concentrated to give an oil. Toluene (10 mL) and ethanol (10 mL) were added and the mixture was concentrated to give 1.79 g of brownish, very hygroscopic solid. The solid was suspended in ethyl acetate and stirred vigorously for 12 h. The solids were quickly collected by filtration and dried under high vacuum to give the product (81%) as a colorless solid. |
|
With hydrogenchloride; water; In tetrahydrofuran; for 1h; |
Example 18 Example 18 provides a method for the preparation of 3-alkoxypyrrolidines from N-Boc-3-hydroxypyrrolidine. 1-Boc-3-hydroxypyrrolidine (16.1 mmol) was added in portions to a suspension of sodium hydride (22.0 mmol) in tetrahydrofuran (40 mL) at 0 C. The reaction mixture was diluted with tetrahydrofuran (60 mL) and allowed to warm to rt. Methyl iodide (21.0 mmol) was added to the cloudy suspension after 1 h and the reaction mixture was maintained at rt for 6 h. The reaction mixture was concentrated and re-dissolved in ethyl acetate (100 mL). The extract was washed with saturated ammonium chloride (20 mL), water (20 mL), and brine (20 mL) and was dried (sodium sulfate). The residue was purified by chromatography (1/4 ethyl acetate/hexane) to give the ether. The iV-Boc product was dissolved in tetrahydrofuran (30 mL) and 6 N hydrochloric acid (20 mL) was added. The resultant mixture was stirred for 1 h and was concentrated to give an oil. Toluene (10 mL) and ethanol (10 mL) were added and the mixture was concentrated to give 1.79 g of brownish, very hygroscopic solid. The solid was suspended in ethyl acetate and stirred vigorously for 12 h. The solids were quickly collected by' filtration and dried under high vacuum to give the product (81%) as a colorless solid. An alternative procedure used for the removal of the N-Boc groups entails exposure to trifluoroacetic acid for 4 h. followed by concentration of the reaction mixture. This procedure may be useful for the production of the amine as a free base.The following amine was prepared using this procedure:3-Methoxypyrrolidine hydrochloride. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping