|
With sulfuric acid; nitric acid; In water; at -10 - -5℃; for 0.25h; |
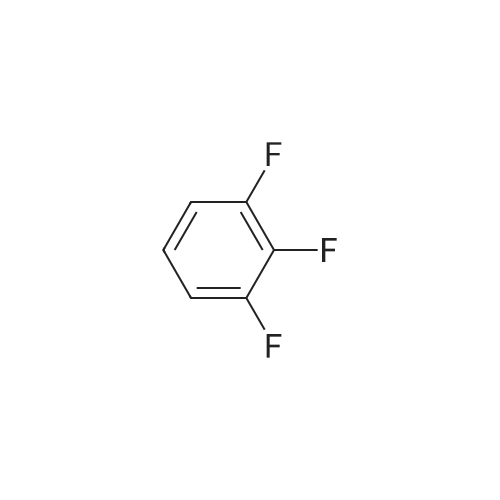
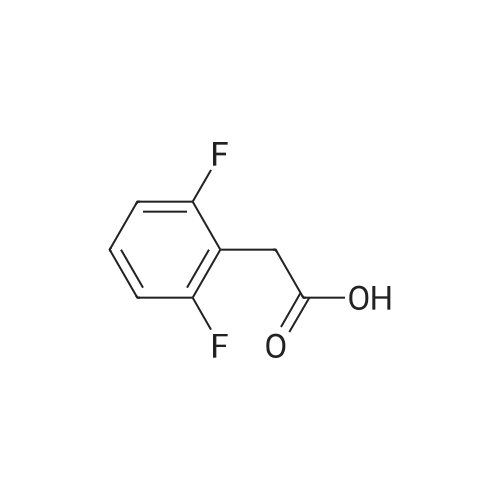
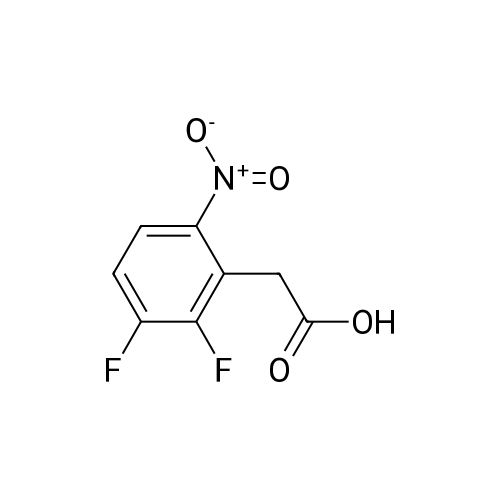
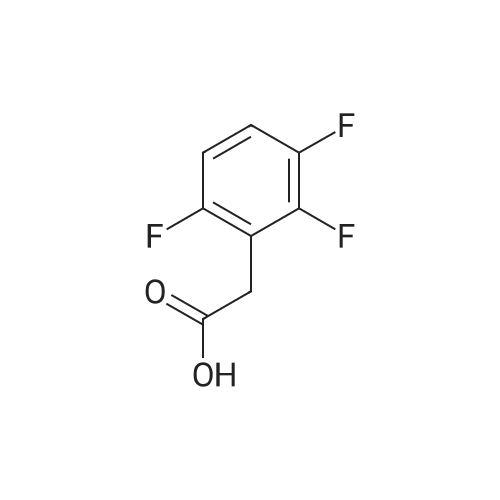
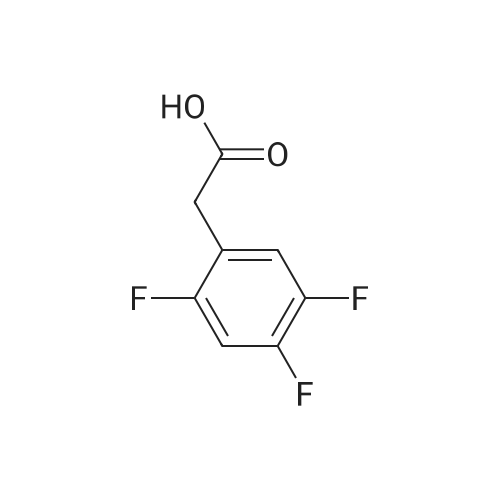
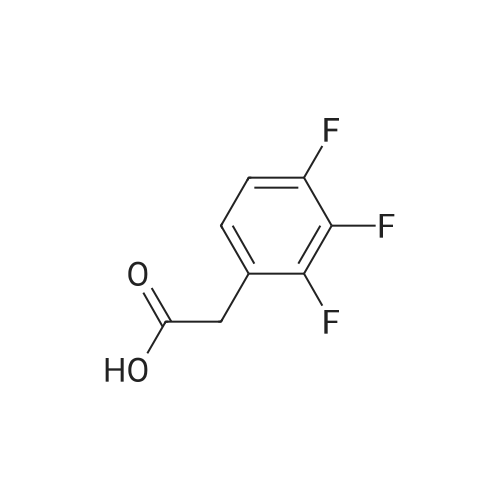
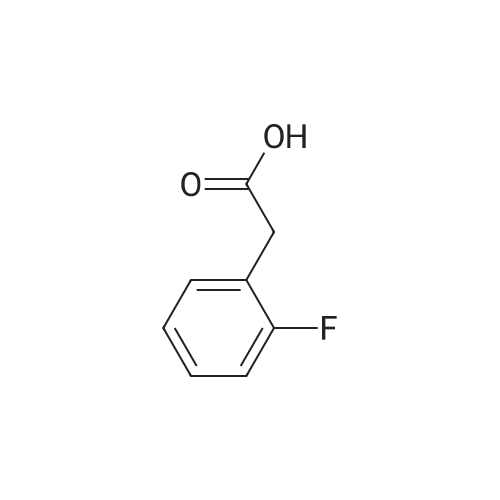
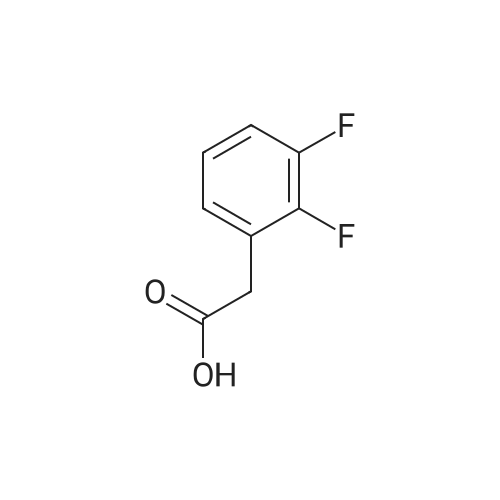
Step 1: Preparation of (2,3-difluoro-5-nitrophenyl)acetic acid <strong>[145689-41-4](2,3-Difluoro-phenyl)-acetic acid</strong> (5 g, 0.0290 mol) is dissolved in concentrated sulfuric acid (20 ml) and the resulting solution cooled to -10 C. with vigorous stirring. A solution of nitric acid (1.88 ml, 69.3%, 0.0290 mol) and sulfuric acid (2 ml) is added dropwise at a rate such that the temperature remains below -5 C. The thickened slurry is stirred for 15 minutes and then poured on ice. The resulting white precipitate is filtered and dried under vacuum (6.3 g, 99%) and consists of a 50/50 mixture of 5 and 6-NO2 regioisomers suitable for use directly in the next step. |
|
With sulfuric acid; nitric acid; In concentrated sulfuric acid; at -10 - -5℃; for 0.25h; |
Step 1a: Preparation of (2,3-difluoro-5-nitrophenyl)acetic acid (12) <strong>[145689-41-4](2,3-Difluoro-phenyl)-acetic acid</strong> (11, 5 g, 0.0290 mol) is dissolved in concentrated sulfuric acid (20 ml) and the resulting solution cooled to -10 C. with vigorous stirring. A solution of nitric acid (1.88 ml, 69.3%, 0.0290 mol) and sulfuric acid (2 ml) is added dropwise at a rate such that the temperature remains below -5 C. The thickened slurry is stirred for 15 minutes and then poured on ice. The resulting white precipitate is filtered and dried under vacuum (6.3 g, 99%) and consists of a 50/50 mixture of 5 and 6-NO2 regioisomers suitable for use directly in the next step. |
|
With sulfuric acid; nitric acid; In water; at 0℃; for 0.416667h; |
Example 15 Preparation of N-[(5S)-3-(9-fluoro-l-methyl-2-oxo-l,2,4,5-tetrahydro-3,l- benzoxazepin-7-yl)-2-oxo-l,3-oxazolidin-5-yl]methyl}acetamideStep 1: Preparation of {2-[benzyl(methyl)amino] -3 -fluoro-5-nitrophenyl} acetic acid.; To a cold (-100C) solution of <strong>[145689-41-4](2,3-difluorophenyl)acetic acid</strong> (40.0 g, 232 mmol) in concentrated sulfuric acid (120 mL) is added a cold mixture (00C) of nitric acid (21 mL, 70% <n="38"/>aqueous, 0.33 mol) and concentrated sulfuric acid (40 mL) over 5 min. After 20 min, the mixture is poured into ice water (1 L) and extracted with CH2Cl2 (3x300 mL). The combined organic layers are washed by brine, dried (Na2SO4), filtered and concentrated to provide a mixture of (2,3-difluoro-5-nitrophenyl)acetic acid and (2,3-difluoro-6-nitrophenyl)acetic acid (95%). This mixture is directly used in the next step without further purification. To a solution of the above regioisomeric mixture (52 g, 0.24 mol) in DMSO (100 mL) is added N- benzylmethylamine (175 mL, 1.36 mol). The mixture is stirred at 800C for 17 h, cooled to 23C, diluted with aqueous sodium hydroxide (30 g, in 1 L H2O) and extracted with ether (3x300 mL). The aqueous layer is then acidified with 12 M aqueous HCl to pH = 4 and extracted with CH2Cl2 (4x300 mL). The combined CH2Cl2 extracts are washed by brine, dried (Na2SO4), filtered and concentrated to afford the title compound. 1H NMR (300 MHz, CDCl3): 2.64 (s, IH), 2.70 (s, 3H), 3.82 (s, 2H), 4.15 (d, J =1.8 Hz, 2H), 7.27 (m, 5H), 7.90 (dd, J =2.7 Hz, J =11.4 Hz, IH), 7.95 (m, IH). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping