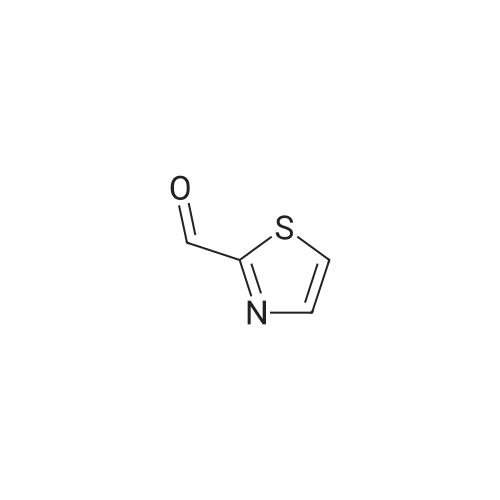
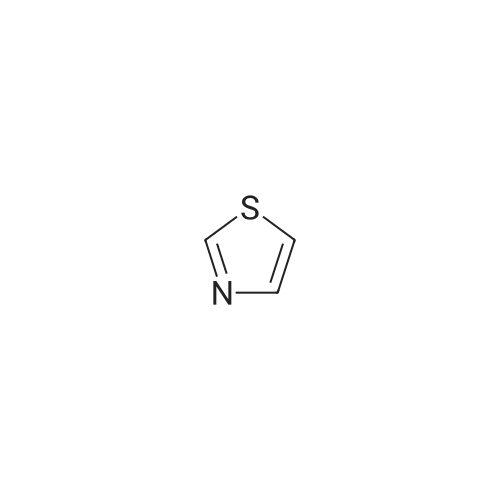
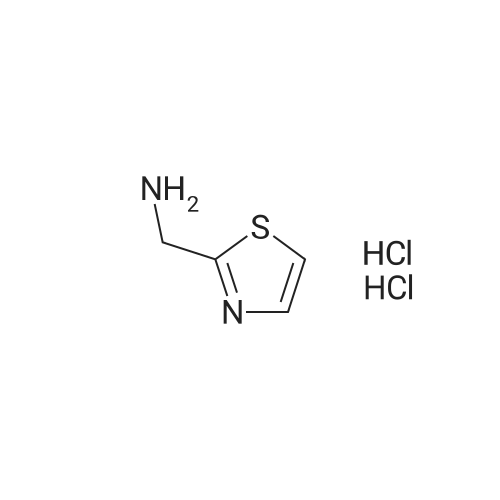
| 74% |
at -60℃; for 2 h; |
A stirred and cooled (-60 0C) sol. of thiazole-2-carbaldehyde (3.97 g, 35.1 mmol) in MeOH (35 mL) was treated with NaBH4 (1.33 g, 35.1 mmol). The reaction mixture was stirred at -60 0C for 2 h, then carefully quenched with acetone (2.7 mL), warmed to rt, and the solvents were removed in vacuo. Purification of the crude by FC (EtOAc) yielded the title compound (3.00 g, 74percent) as an orange oil that crystallized at -20 0C and remained a solid upon warming to rt. |
| 66% |
Stage #1: With sodium tetrahydroborate In methanol at 0℃;
Stage #2: With water In methanol |
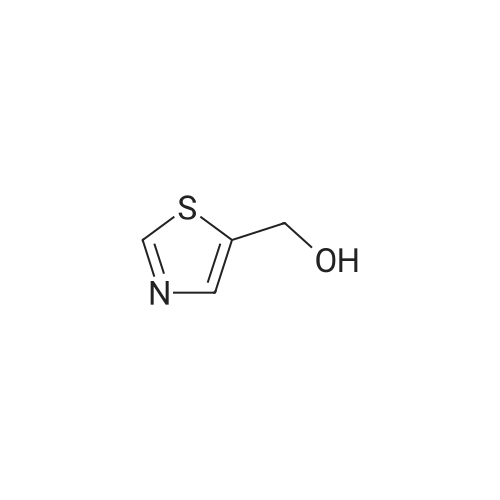
Referential Example 12: thiazole-2-methanol
While stirring under ice cooling, sodium borohydride (242 mg, 6.40 mmol) was added to a methanol (10 ml) solution of 2-formylthiazole (483 mg, 4.27 mmol)..
After completion of the reaction was confirmed, water was added to the reaction mixture..
The resulting mixture was concentrated under reduced pressure..
Water and ethyl acetate were added to the residue to separate the organic layer..
The organic layer was washed with brine and then dried over anhydrous sodium sulfate..
After filtration, the filtrate was concentrated under reduced pressure..
The residue was subjected to chromatography on a silica gel column..
The fraction obtained from the hexane:ethyl acetate (=1:1) elude was concentrated under reduced pressure, whereby the title compound (324 mg, 66percent) was obtained as a white solid.1H-NMR (400MHz, CDCl3) δ: 3.30-3.70(1H,m), 5.14(2H,s), 7.32(1H,d,J=3.4Hz), 7.74(1H,d,J=3.2Hz). MS (m/z): 116 (M++H).
|
| 62% |
With sodium hydroxide; sodium tetrahydroborate In methanol |
b.
2-(Hydroxymethyl)thiazole
To a cooled solution (0° C.) of 2-formylthiazole (described in example 71a) (1.50 g, 13.27 mmol) in methanol (25 mL) under nitrogen was added sodium borohydride (0.300 g, 7.94 mmol).
After the addition was complete, the reaction was warmed to room temperature over 1 h and stirred at room temperature for 3 h.
Excess reagent was quenched with acetone (10 mL) and stirred 18 h.
The reaction was acidified with 3N HCl (25 mL), cooled to 0° C. and rebasified with 2.5N NaOH (40 mL).
The aqueous phase was extracted with ethyl acetate (3*25 mL).
Combined organic extracts were dried over anhydrous magnesium sulfate, filtered and reduced to a relatively pure oil in 62percent yield. TLC analysis (Rf 0.15, 40percent ethyl acetate in hexane).
No further purification was required. MS (CI, CH4) m/z 116 (M+1,82), 144 (M+29,17), 98 (100).
|
| 60% |
With sodium borohydrid In methanol |
Example 391A
1,3-thiazol-2-ylmethanol
To thiazole-2-carbaldehyde (1133 mg, 1 mmol) in methanol (10 mL) was added sodium borohydride (41 mg, 1.2 mmol) portion wise at 0° C.
The mixture was stirred at room temperature for 2 hours.
The mixture was diluted with water and acidified to pH 3 with 1M hydrochloric acid, and extracted with ethyl acetate (2*50 mL).
The organic layers were washed with saturated aqueous sodium bicarbonate, water, brine, dried over magnesium sulfate, filtered and concentrated to give the title compound (69 mg, 60percent).
|
| 58% |
With sodium tetrahydroborate In methanol at 0℃; for 3 h; |
Aldehyde 3 (5.8 g, 51.3 mmol, 1 eq)/10 mL methanol was treated with NaBH4 (2.1 g,1.1 eq) at 0 °C for 3 h. The solvent was removed and EtOAc was added forextraction. Flash chromatography (2:1 Hexane : EtOAc) provided 3.4 g of the desiredproduct (58 percent yield).1H NMR (CDCI3 8): 4.90 (s, 2H) 7.27 (m, 1H) 7.70
|
| 56% |
Stage #1: With sodium tetrahydroborate In ethanol for 16 h; Heating / reflux
Stage #2: With water In ethyl acetate |
0.20 ml (2.28 mmol) 2-thiazolecarboxaldehyde are placed in 10 ml of ethanol, 0.172 g (4.55 mmol) sodium borohydride are added.
The reaction mixture is refluxed for 16 hours with stirring, then concentrated by evaporation.
The residue is extracted with water and ethyl acetate, the organic phase is washed with water.
The combined aqueous phases are saturated with sodium chloride and extracted with ethyl acetate.
The resulting organic phase is dried and evaporated to dryness.
Yield: 0.146 g (=56percent of theoretical)
|
| 56% |
Stage #1: With sodium tetrahydroborate In ethanol at 20℃; for 16 h; Heating / reflux
Stage #2: With water In ethyl acetate |
Precursor for compound 17: thiazol-2-yl-methanol
0.20 ml (2.28 mmol) 2-thiazol-carboxaldehyde are placed in 10 ml of ethanol at ambient temperature, 172 mg (4.55 mmol) sodium borohydride are added and then the mixture is refluxed for 16 hours with stirring.
Then the reaction mixture is concentrated by evaporation, the residue is extracted with water and ethyl acetate.
The aqueous phase is saturated with sodium chloride and extracted again with ethyl acetate.
The resulting organic phase is dried and evaporated to dryness. Yield: 146 mg (=56percent of theoretical)
|
| 53% |
Stage #1: With sodium tetrahydroborate In ethanol at 20℃; for 1.5 h;
Stage #2: With water In ethanol |
Sodium borohydride (0.17 g, 4.49 mmol) was added to a solution * of 2- thiazolecarboxaldehyde (0.39 mL, 4.44 mmol) in ethanol (25 mL). After stirring for 1.5 h at room temperature, the reaction was quenched with water and concentrated. The residue was dissolved in ethyl acetate and washed with water and brine, dried over magnesium sulfate and concentrated to give 0.27 g, (53percent) of thiazol-2-yl-methanol as a tan oil which had: NMR (CDCI3) δ 7.72 (d, J = 3.3 Hz, 1 H), 7.31 (d, J = 3.3 Hz, 1 H), 4.95 9s, 2H); 2.83 (br s, 1 H). This was mixed with triethyl amine (0.33 mL, 2.37 mmol) in methylene chloride (4 mL) and cooled in ice. Methanesulfonylchloride (0.18 mL, 2.33 mmol) in methylene chloride (2mL) was added dropwise over 1 min. After 30 min stirring at O0C, the reaction was washed with water and brine, dried over magnesium sulfate and concentrated to yield 0.41 g (91 percent) of methanesulfonic acid thiazol-2-ylmethyl ester as an orange oil which had: NMR (CDCI3) δ 7.83 (d, J = 3.3 Hz, 1 H), 7.45 (d, J = 3.3 Hz, 1 H), 5.50 (s, 2H), 3.06 (s, 3H). |
| 16.4% |
With sodium tetrahydroborate In methanol at -60℃; for 1 h; Inert atmosphere |
To a solution of n-BuLi (8.4 ml, 1.6 mol/l, 13.4 mmol) in THF (30 mL) was added 2-bromothiazole (377 mg, 2.12 mmol) dropwise under nitrogen atmosphere at -70° C., and the mixture was stirred at the temperature for 1 h.
Then DMF (1.4 ml, 18.3 mmol) was added into the solution dropwise under nitrogen atmosphere at -70° C.
The resulting mixture was stirred at the temperature for 1 h.
Then the mixture was quenched with aqueous saturated ammonium chloride, diluted with ethyl acetate and water, and the phases were separated.
The organic phase was washed with brine, dried over sodium sulfate, filtered and concentrated to give yellow oil.
The yellow oil was dissolved in methanol (15 ml), cooled to -60° C., and sodium borohydride (463 mg, 12.2 mmol) was added portionwise under nitrogen atmosphere.
The mixture was stirred at the temperature for 1 h.
The reaction was quenched with acetone and concentrated.
The residue was diluted with ethyl acetate and water, and the phases were separated.
The organic layer was dried over sodium sulfate, filtered and concentrated, then purified by silica gel chromatography eluting with petroleum/ethyl acetate=3:1 to give thiazol-2-ylmethanol (230 mg, 16.4percent yield) as brown oil. LCMS MH+ 116. |
| 15.2% |
With sodium tetrahydroborate In methanol at 0℃; for 2 h; |
To a solution of 18a (20.55 g, 0.18 mol) in MeOH (100 mL) was added NaBH4 (7.6 g, 0.20 mol) at 0°C. The reaction mixture was stirred at 0°C for 2 hours and monitored using TLC. Then, acetone (20 mL) was added into the solution and stirred for 20 minutes and the mixture was concentrated in vacuo. After that, the residue was washed with water (200 mL) and extracted with ethyl acetate (300 mL), washed with brine and dried over anhydrous sodium sulfate. After concentration in vacuo, and the resutling residue was purified using Si02 chromatography, eluting with petroleum ether: ethyl acetate (10/1-1/1) to provide 18b (3.18 g, 15.2percent) |
| 15.2% |
With sodium tetrahydroborate In methanol at 0℃; for 2 h; |
To a solution of 18a (20.55 g, 0.18 mol) in MeOH (100 mL) was added NaBH4 (7.6 g, 0.20 mol) at 0°C. The reaction mixture was stirred at 0°C for 2 hours and monitored using TLC. Then, acetone (20 mL) was added into the solution and stirred for 20 minutes and the mixture was concentrated in vacuo. After that, the residue was washed with water (200 mL) and extracted with ethyl acetate (300 mL), washed with brine and dried over anhydrous sodium sulfate. After concentration in vacuo, the residue was purified using Si02 chromatography, eluting with petroleum ether: ethyl acetate (10/1-1/1) to provide 18b (3.18 g, 15.2percent). |
| 12 g |
With sodium tetrahydroborate In methanol at 0 - 20℃; |
To a mixture of 2-formylthiazole (84.22g, 0.75mol) and methanol (100 mL) was added sodium borohydride (120g, 1.5mol) at 0°C, which was stirred for overnight at room temperature. Water was added to this reaction mixture, which was then extracted with ethyl acetate. The organic layer was separated, washed with saturated aqueous sodium chloride, dried over anhydrous magnesium sulfate, and filtered. The filtrate was concentrated under a reduced pressure, and the residue was purified by silica gel column chromatography with DCM/MeOH (100:1-50:1) to afford thiazol-2-ylmethanol as light yellow oil (12 g ,14percent). |
| 230 mg |
at -60℃; for 1 h; Inert atmosphere |
Step 1 thiazol-2-ylmethanol To a solution of n-BuLi (8.4 ml , 1.6 mol/1 , 13.4 mmol) in THF (30 mL) was added 2- bromothiazole (377 mg, 2.12 mmol) dropwise under nitrogen atmosphere at -70 °C, and the mixture was stirred at the temperature for 1 h. Then DMF (1.4 ml, 18.3 mmol) was added into the solution dropwise under nitrogen atmosphere at -70 °C. The resulting mixture was stirred at the temperature for 1 h. Then the mixture was quenched with aqueous saturated ammonium chloride, diluted with ethyl acetate and water, and the phases were separated. The organic phase was washed with brine, dried over sodium sulfate, filtered and concentrated to give yellow oil. The yellow oil was dissolved in methanol (15 ml) , cooled to -60 °C, ans sodium borohydride (463 mg , 12.2 mmol) was added portionwise under nitrogen atmosphere. The mixture was stirred at the temperature for 1 h. The reaction was quenched with acetone and concentrated. The residue was diluted with ethyl acetate and water, and the phases were separated. The organic layer was dried over sodium sulfate, filtered and concentrated, then purified by silica gel chromatography eluting with petroleum/ ethyl acetate= 3: 1 to give thiazol-2-ylmethanol (230 mg, 16.4 percent yield) as brown oil . LCMS MH+ 1 16. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping