| 65% |
With potassium carbonate; triethylamine; In acetonitrile; for 12h;Reflux; |
Compound 3 (233 g, 1.0mol) and 6 (245 g, 1.0 mol) were dissolved in acetonitrile (3 L), and then, triethylamine (101 g, 1.0mol) and K2CO3 (138g,1.0mol) were added to the solution. The mixture was heated at reflux for 12 h. Subsequently, the reaction mixture was poured into cold water (3L). After filtration, the solid was dissolved in EtOAc, and then, HCl-EtOAc saturated solution was added to enable crystallization. Product 7 was obtained as a white solid. Yield 287 g (65%) with 99% HPLC purity after the mixture was filtered. m.p. 276.5-279.2C (Lit1 277-279C). 1H NMR (400MHz, DMSO-d6): delta 11.35 (s, 1H), 8.04 (d, J=18.3Hz, 2H), 7.74-6.88 (m, 8H), 3.69-3.15 (m, 8H), 2.74 (t, J= 7.2Hz, 2H), 2.37 (t, J =7.2Hz, 2H), 1.80-1.61 (m, 2H), 1.54 (d, J=6.8Hz, 2H). 13C NMR (100 MHz, DMSO-d6): delta 159.9, 148.8, 147.9, 138.6, 137.7, 127.8, 126.9, 124.7, 124.0, 123.3, 120.9, 117.8, 115.7, 112.4, 111.6, 109.5, 107.3, 100.0, 57.5, 52.8(2), 49.8(2), 27.7, 26.1, 24.1. HRMS (ESI): m/z [M+H]+ calculated for C26H27N5O2: 442.2238, found: 442.2240. |
| 44 g |
|
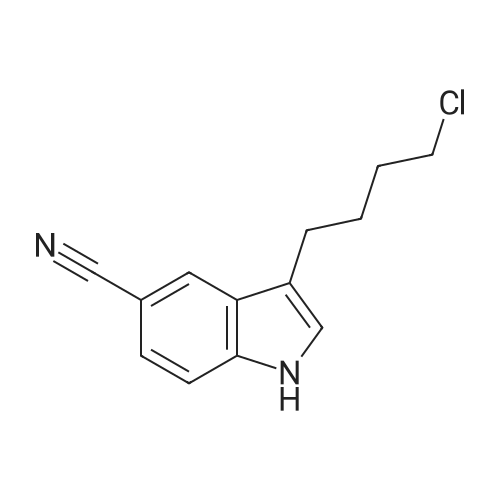
Example 1 : Preparation of 5- |4-[4-r5-Cyano- lH-Indol-3-yf)ButyllPiperazin-l-yl}-l- Benzofuran-2-Carboxamide 3-(4-Chlorobutyl)-lH-indole-5-carbonitrile (34.2 g) was added to acetonitrile (300 mL). Sodium iodide (33.1 g) was added to the reaction mixture. The reaction mixture was heated to 80C to 85C and maintained for 60 minutes. The reaction mixture was cooled to 60C. 5-(Piperazin-l -yl)-l-benzofuran-2-carboxamide (30 g) and triethylamine (18.6 g) were added to the reaction mixture. The reaction mixture was heated to 80C to 83C for 18 hours. The reaction mixture was cooled to 20C to 30C. The reaction mixture was added to water (300 mL), dichloromethane (300 mL), and sodium thiosulphate (7.5 g). Concentrated hydrochloric acid (15 mL) was added to the reaction mixture and stirred for 1.5 hours at 20C to 30C. The solid obtained was filtered and washed with dichloromethane (80 mL) and deionized water (150 mL). The reaction mixture was added to ethyl acetate (600 mL) and water (300 mL). Triethyl amine (20 g) was added to the reaction mixture. The reaction mixture was heated to 70C to 75C. The layers obtained were separated and the organic layer was washed with water (100 mL). The organic layer was recovered under vacuum. 2-Propanol (60 mL) was added to the reaction mixture and stirred at 20C to 30C for 1 hour. The reaction mixture was filtered, washed with 2-propanol (30 mL) and dried under vacuum at 45C to 50C for 12 hours to obtain the title compound having XRPD as depicted in Figure 1. Yield: 44.0 g. |
|
With triethylamine; In acetonitrile; at 35 - 80℃; for 48h; |
Acetonitrile (500 ml), 5-(l-piperazinyl) benzofuran-2-carboxamide of Formula (G) (100 g), 3-(4-chlorobutyl)-lH-indole-5-carbonitrile of Formula (H) and Triethyl amine (200 g) were added to a four-neck two litre round bottom flask at 35C and reaction mass was heated to 80C and maintained for 48 hours. The reaction mass was cooled to 55C to afford vilazodone of Formula (1). |
|
With N-ethyl-N,N-diisopropylamine; In 1-methyl-pyrrolidin-2-one;Heating; |
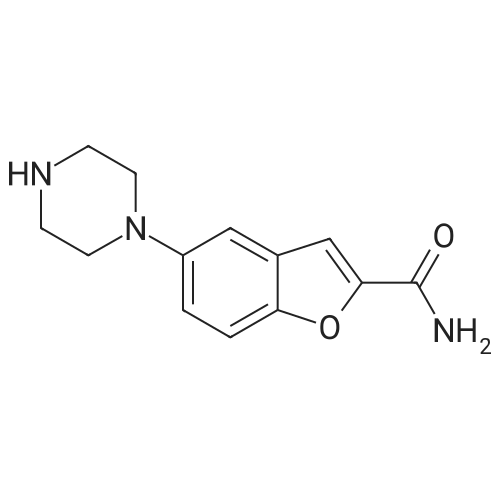
3-(4-chlorobutyl)-1H-indole-5-carbonitrile (1.01 g, 1.0 equiv.), <strong>[183288-46-2]5-(piperazin-1-yl)benzofuran-2-carboxamide</strong> (1.10 g, 1.03 equiv.), diisopropylethyl amine (DIPEA, 0.64 g, 1.16 equiv,), and N-Methyl-2-pyrrolidone (NMP, about 40 mL) are charged to a suitable vessel. It is to be noted that 5-(piperazin-1-yl) benzofuran-2-carboxamide may be produced using methods known in the art (see, e.g., U.S. Patent No. 5,977,112). The mixture is heated and stirred until the reaction is complete. Acetonitrile (about 40 mL) and water (about 40 mL) are charged while maintaining elevated temperature. The mixture is cooled slowly to precipitate vilazodone, filtered, and the wet cake is washed with acetonitrile/water and then with acetonitrile. The wet product is dried to provide crude vilazodone. Purification is carried out via conventional means known in the art, e.g., filtration and precipitation. |
| 7 g |
With potassium carbonate; In water; toluene; at 90 - 95℃; for 29h; |
Example 3: Preparation of vilazodone free basePotassium carbonate (2.8 g) was added to water (25 mL) and stirred for 10 minutes. 5-Piperazin- 1 -ylbenzofuran-2-caboxamide (5.0 g), 3 -(4-chlorobutyl)- 1 H-indole25 5-carbonitrile (5.7 g), and toluene (25 mL) were added to the reaction mixture and thereaction mixture was heated to 90C to 95C for 29 hours. The reaction mixture was cooled to 3 0C, filtered, and washed with water (25 mL). The solid obtained was dried at 45C to 50C to obtain the title compound.Yield: 7.0 g |
| 2.8 mg |
|
-(Piperazin-l-yl)-l-benzofuran-2-carboxamide (5 g; prepared according toExample 2) and 3-(4-chloro butyl)-lH-indole-5-carbonitrile (5.7 g) were added to N-methyl pyrollidone (25 mL). The temperature of the reaction mixture was increased to 110C to 120C and stirred for 2 hours. Tributyl amine (3.77 g) was added to the reaction mixture and stirred at 110C to 120C for 4 hours. The reaction mixture was cooled to 20C to 35C. Hydrobromic acid (2.5 mL) and 2-propanol (50 mL) were added to the reaction mixture. Vilazodone hydrobromide seed (0.005 g) was added to the reaction mixture and stirred at 0C to 5C for 1 hour. The reaction mixture was filtered, and then washed with cooled 2-propanol (25 mL). The reaction mixture was added to a mixture of N-methyl pyrollidone (25 mL) and hydrobromic acid (0.5 mL). 2-Propanol (50 mL) was added to the reaction mixture and stirred for 2 hours. The reaction mixture was filtered and washed with 2-propanol (10 mL). The reaction mixture was added to 2-propanol (100 mL) and deionized water (50 mL). The temperature of the reaction mixture was increased to 80C to 82C. Activated carbon (1 g) was added to the reaction mixture and stirred for 1 hour. The reaction mixture was filtered and washed with 2-propanol (20 mL) and deionized water (10 mL). The pH of the filtrate was adjusted to 7.2 with 7% sodium bicarbonate solution (9 mL). The reaction mixture was filtered and washed with deionized water (20 mL) and 2-propanol (20 mL). The solid obtained was dried in air at 50C to 55C to obtain the title compound. Yield: 2.8 g |
|
With N-ethyl-N,N-diisopropylamine; In dimethyl sulfoxide; at 35 - 80℃; for 30h; |
DMSO (1000 ml), <strong>[183288-46-2]5-(1-piperazinyl)benzofuran-2-carboxamide</strong> of Formula (G) (100 g), 3-(4-chlorobutyl)-1H-indole-5-carbonitrile of Formula (H) and diisopropylethylamine (DIPEA) (1500 ml) were added to a four-neck two litre round bottom flask at 35 C. and reaction mass was heated to 80 C. and maintained for 30 hours. The reaction mass was cooled to 55 C. The reaction mass was treated with EDTA (3 g) and charcoal (10 g). The residue was filtered and treated with DMSO (800 ml) at 80-85 C. and stirred for 15 minutes. The reaction mass was treated with acetone at 20-25 C. and stirred for 1 hour. The reaction mass was washed with acetone (2*100) and treated with acetic acid (720 ml) and methanol (500 ml) at 25-30 C. and stirred for 10 minutes. The reaction mass is heated to 80-81 C. and treated with activated charcoal (10 g) and stirred for 15-20 min. The reaction mass was cooled to 15-20 C., filtered and washed with methanol (2*100 ml). The product was dried in hot air oven at 60-65 C. for 12-14 hours. The product was further dried in oven at 105 C. for 4 hours afforded Form-Z of vilazodone of Formula (1). (HPLC purity >99.70%). |
| 44 g |
|
3-(4-Chlorobutyl)-1H-indole-5-carbonitrile (34.2 g) was added to acetonitrile (300 mL). Sodium iodide (33.1 g) was added to the reaction mixture. The reaction mixture was heated to 80 C. to 85 C. and maintained for 60 minutes. The reaction mixture was cooled to 60 C. 5-(Piperazin-1-yl)-1-benzofuran-2-carboxamide (30 g) and triethylamine (18.6 g) were added to the reaction mixture. The reaction mixture was heated to 80 C. to 83 C. for 18 hours. The reaction mixture was cooled to 20 C. to 30 C. The reaction mixture was added to water (300 mL), dichloromethane (300 mL), and sodium thiosulphate (7.5 g). Concentrated hydrochloric acid (15 mL) was added to the reaction mixture and stirred for 1.5 hours at 20 C. to 30 C. The solid obtained was filtered and washed with dichloromethane (80 mL) and deionized water (150 mL). The reaction mixture was added to ethyl acetate (600 mL) and water (300 mL). Triethyl amine (20 g) was added to the reaction mixture. The reaction mixture was heated to 70 C. to 75 C. The layers obtained were separated and the organic layer was washed with water (100 mL). The organic layer was recovered under vacuum. 2-Propanol (60 mL) was added to the reaction mixture and stirred at 20 C. to 30 C. for 1 hour. The reaction mixture was filtered, washed with 2-propanol (30 mL) and dried under vacuum at 45 C. to 50 C. for 12 hours to obtain the title compound having XRPD as depicted in fig.1. Yield: 44.0 g. |
|
With sodium acetate; In N,N-dimethyl acetamide; at 90℃; for 18h; |
36.10 g (155 mmol) of 3-(4-chlorobutyl)-5-cyanoindole was added to a 1 L single-necked flask, <strong>[183288-46-2]5-(piperazin-1-yl)benzofuran-2-carboxamide</strong> 36.00 g (147 mmol), 25.50 g of sodium acetate (310 mmol), stirring in 500mL DMAC, the reaction was carried out at 90 C (outside temperature) for 18 hours, the reaction solution was cooled to room temperature, 1 L of water was added, extraction with ethyl acetate (3 x 400 mL), the organic layers were combined, the organic layer was washed with water (2 x 300 mL), saturated sodium chloride solution (200 mL), dried over anhydrous sodium sulfate, filtered and the filtrate was concentrated to give a yellow solid. Using mixed solvent purification, the solid was first dissolved in THF, acetone was added with stirring, methanol, stirring, precipitating solid, filtering, vacuum drying, weighing, vilazodone. |
| 13.2 g |
With triethylamine; sodium bromide; In isopropyl alcohol; for 18h;Reflux; Inert atmosphere; |
Under nitrogen, the above prepared 6.1g <strong>[183288-46-2]5-(piperazin-1-yl)benzofuran-2-carboxamide</strong>, 5.8 g of 3-(4-chlorobutyl-5-cyanoindole, 3.5 g of sodium bromide and 2.8 g of triethylamine were successively dissolved in 100 mL of isopropanol to form a reaction solution. The reaction was stirred at reflux for 18 hours until the reaction was complete by TLC. The reaction mixture was added dropwise to 150 mL of a 5% aqueous solution of sodium carbonate until a light yellow solid precipitated. The reaction mixture was stirred for 1 hour and filtered to obtain a cake. The cake was washed with 100 mL of water once to obtain 11.8 g of vilazodone , Purity 99.1%, yield was 93.1%. |
| 7.25 kg |
With triethylamine; N-ethyl-N,N-diisopropylamine; sodium iodide; In dimethyl sulfoxide; at 25 - 95℃; for 10h;Large scale; |
2. In a 100L enamel kettle,Add 57.75 kg of dimethyl sulfoxide and stir.6.22 Kg of <strong>[183288-46-2]5-(1-piperazinyl)-benzofuran-2-carboxamide</strong> of formula III is added,Add 5.22 kg of 3-(4-chlorobutyl)indole-5-carbonitrile of formula IV,3.51kg anhydrous sodium iodide, 3.57kg N,N-diisopropylethylamine and 4.36kg triethylamine(material volume is about 70L), heat up to 95 C, and keep reacting at 95 C for 10 hours.3-(4-Chlorobutyl)indole-5-carbonitrile was completely reacted to room temperature (25 C).The reaction solution was transferred to a 500 L enamel kettle, 36.75 kg of dichloromethane and 157.5 kg of water were added (material volume: about 280 L), stirred for 2 hours, centrifuged, washed with 5 kg of dichloromethane, and centrifuged at 50 ± 2 C. Drying in the wind for 12 hours,Obtaining 9.25 kg of the crude verazodone shown by formula V,The yield is close to the theoretical value and the purity is 90 to 96%.Reaction monitoring: TLC, dichloromethane: methanol (add 2 to 3 drops of ammonia) = 6:1. 3, refined (1) Recrystallization:The first recrystallization: into a 300L enamel reactor, add 9.25kg of verazodone crude and 23L of DMSO, warmed to 50 C, all solids were dissolved under stirring, and then added 92.5L of acetone to continue to raise the temperature to 60 C, add 462.5 g activated carbon, stirring and decoloring for 1h, suction filtration, cooling, adding 92.5L water under stirring, a large amount of solids are precipitated, centrifuged, rinsed with 15L water, blast at 50 CDry for 15~20h, weighing 8.5kg;Second recrystallization: into a 50L reactor, add 8.5kg of verazolonone and 21.1L of DMSO, raise the temperature to 50 C, dissolve all the solids under stirring, add 85L of acetone and continue to raise the temperature to 60 C, add 422.5g Activated carbon, stirring and decoloring for 1h, suction filtration, cooling, adding 85L of water under stirring, a large amount of solids are precipitated, centrifuged, rinsed with 15L of water, blasted at 50 C, saidThe weight is 7.65kg;The third recrystallization: into a 50L reactor, 7.65g of verazosone and 19.15L of DMSO were added, the temperature was raised to 50 C, the solid was completely dissolved under stirring, and 76.5 L of acetone was added to continue heating to 60 C, and 382.5 was added. g activated carbon, stirring and decoloring for 1h, suction filtration, cooling, adding 76.5L water under stirring, a large amount of solids are precipitated, centrifuged, rinsed with 15L water, blasted at 50 C, weighed 7.25kg; |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping