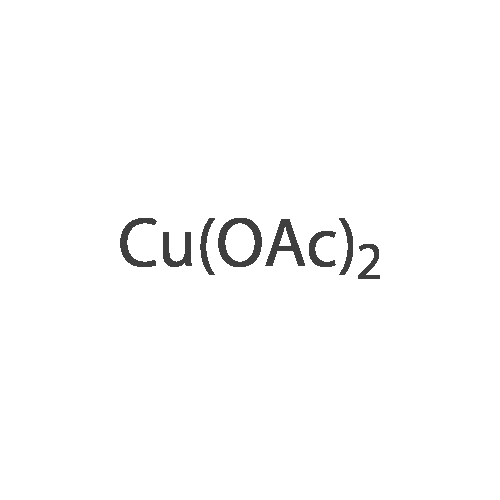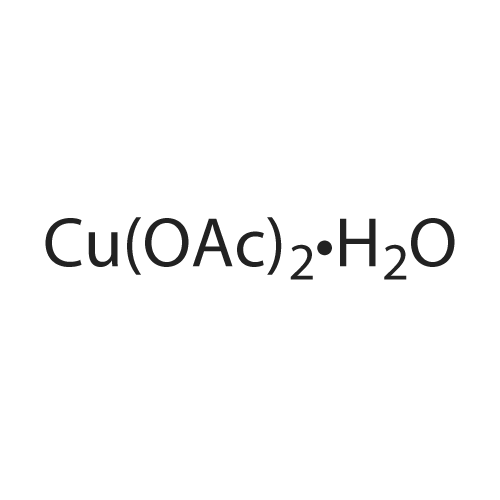Alternatived Products of [ 14172-91-9 ]
Product Details of [ 14172-91-9 ]
| CAS No. : | 14172-91-9 |
MDL No. : | MFCD00148862 |
| Formula : |
C44H28CuN4
|
Boiling Point : |
No data available |
| Linear Structure Formula : | Cu(C4H2NCC6H5)4 |
InChI Key : | - |
| M.W : |
676.27
|
Pubchem ID : | - |
| Synonyms : |
|
Application In Synthesis of [ 14172-91-9 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 14172-91-9 ]
- 1
-
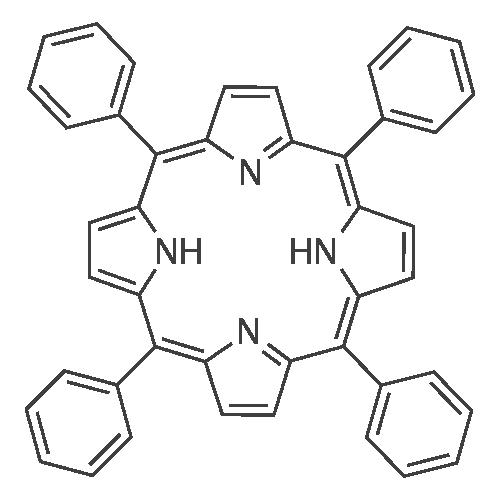 [ 917-23-7 ]
[ 917-23-7 ]

-
copper(II) perchlorate hexahydrate
[ No CAS ]

-
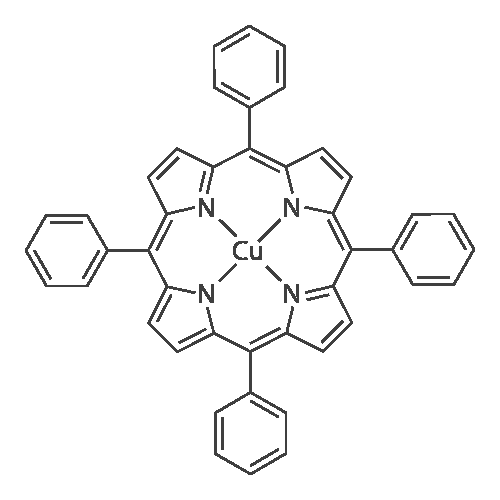 [ 14172-91-9 ]
[ 14172-91-9 ]
Reference:
[1]Inorganic Chemistry,1982,vol. 21,p. 2323 - 2327
[2]Inorganic Chemistry,1982,vol. 21,p. 2323 - 2327
[3]Inorganic Chemistry,1982,vol. 21,p. 2323 - 2327
[4]Inorganic Chemistry,1982,vol. 21,p. 2323 - 2327
[5]Inorganic Chemistry,1982,vol. 21,p. 2323 - 2327
[6]Inorganic Chemistry,1982,vol. 21,p. 2323 - 2327
[7]Inorganic Chemistry,1982,vol. 21,p. 2323 - 2327
[8]Inorganic Chemistry,1982,vol. 21,p. 2323 - 2327
[9]Inorganic Chemistry,1982,vol. 21,p. 2323 - 2327
[10]Inorganic Chemistry,1982,vol. 21,p. 2323 - 2327
[11]Inorganic Chemistry,1982,vol. 21,p. 2323 - 2327
[12]Inorganic Chemistry,1982,vol. 21,p. 2323 - 2327
[13]Journal of Physical Chemistry,1992,vol. 96,p. 1204 - 1215
- 2
-
 [ 917-23-7 ]
[ 917-23-7 ]

-
copper(II) choride dihydrate
[ No CAS ]

-
 [ 14172-91-9 ]
[ 14172-91-9 ]
- 3
-
 [ 917-23-7 ]
[ 917-23-7 ]

-
copper dichloride
[ No CAS ]

-
 [ 14172-91-9 ]
[ 14172-91-9 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With sodium carbonate; In water; at 350.0℃; for 0.0666667h;Inert atmosphere; Sealed tube; |
Using a 50 mL volumetric flask, 1.208 g of copper sulfate as a metal salt was dissolved in 50 mL of distilled water to prepare a 0.1 mol / L CuSO 4 aqueous solution. Next, 0.02 g (3.3 × 10 -5 mol) of tetraphenylporphyrin (TPP) as a compound having a porphyrin-type skeleton, 0.02 g (3.3 × 10 -5 mol) of copper sulfate Aqueous solution of sodium carbonate and 0.032 g of sodium carbonate equivalent to copper sulfate to prevent corrosion of the reaction vessel, and the interior of the reaction vessel was purged with argon and sealed. Next, the reaction vessel was charged into the sand bath set at 350 C. The reaction temperature in the reaction vessel reached the reaction temperature in about 4 minutes. |
Reference:
[1]Doklady Physical Chemistry,1983,vol. 271,p. 510 - 512
Dokl. Phys. Chem. (Transl. of Dokl. Akad. Nauk.),1983,vol. 271,p. 650 - 652
[2]Russian Journal of Coordination Chemistry,2006,vol. 32,p. 276 - 281
[3]Kinetics and Catalysis,1989,vol. 29,p. 674 - 680
[4]Kinetics and Catalysis,1989,vol. 29,p. 674 - 680
[5]Kinetics and Catalysis,1989,vol. 29,p. 674 - 680
[6]Kinetics and Catalysis,1989,vol. 29,p. 674 - 680
[7]Patent: JP5823988,2015,B2 .Location in patent: Paragraph 0068
- 4
-
 [ 917-23-7 ]
[ 917-23-7 ]

-
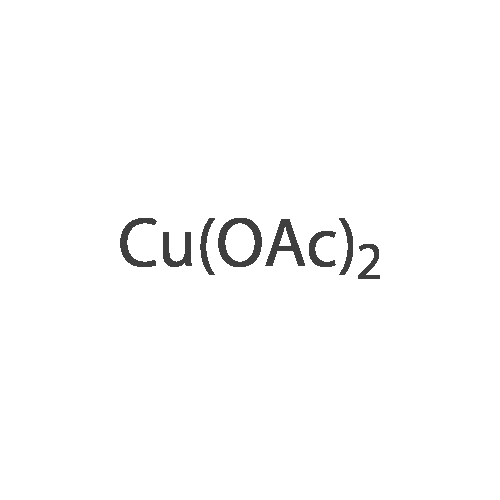 [ 142-71-2 ]
[ 142-71-2 ]

-
 [ 14172-91-9 ]
[ 14172-91-9 ]
| Yield | Reaction Conditions | Operation in experiment |
| 400 mg |
With acetic acid; In chloroform; for 2h;Reflux; |
Copper tetraphenyl porphyrin was synthesised by taking tetra phenyl porphyrin[H2(TPP)]16(500mg) in chloroform(100ml).Copper(II) acetate(200mg) in glacial aceticacid(50ml) was added to the above solution andthe mixture was refluxed for 2hrs. The contents wereconcentrated to a volume of about 50-60ml andcooled to room temperature which resulted in crudecopper-tetraphenyl porphyrin Cu(TPP) (about450mg). The crude product was purified by columnchromatography using neutral alumina andchloroform as eluent. On elution the unreactedtetraphenyl porphyrin was eluted out first, followedby pure Cu(TPP). The chloroform fraction containingCu(TPP) was concentrated to obtain pure crystalsof Cu(TPP)[2]. The formation of Cu(TPP) wasmonitored by UV-visible spectroscopy which givepeaks-around 580, 541 and 417nm respectivelyconfirming the formation of Cu(TPP) (yield=400mg). |
Reference:
[1]Doklady Physical Chemistry,1983,vol. 271,p. 510 - 512
Dokl. Phys. Chem. (Transl. of Dokl. Akad. Nauk.),1983,vol. 271,p. 650 - 652
[2]Thesis Clark Univ., Worcester, Mass., 1957, S. 34,
[3]Gmelin Handbuch der Anorganischen Chemie,Gmelin Handbook: Cu: MVol.B4, 141, page 1757 - 1760
[4]Journal of the American Chemical Society,1948,vol. 70,p. 1808 - 1812
[5]Journal of Thermal Analysis and Calorimetry,1998,vol. 54,p. 243 - 248
[6]Russian Journal of Inorganic Chemistry,2003,vol. 48,p. 439 - 442
[7]Journal of the American Chemical Society,1948,vol. 70,p. 1808 - 1812
[8]Journal of Thermal Analysis and Calorimetry,1998,vol. 54,p. 243 - 248
[9]Journal of Physics and Chemistry of Solids,1988,vol. 49,p. 315 - 322
[10]Journal of the American Chemical Society,1951,vol. 73,p. 4315 - 4320
[11]Gmelin Handbuch der Anorganischen Chemie,Gmelin Handbook: Cu: MVol.B4, 141, page 1757 - 1760
[12]Gmelin Handbuch der Anorganischen Chemie,Gmelin Handbook: Cu: MVol.B4, 141, page 1757 - 1760
[13]Gmelin Handbuch der Anorganischen Chemie,Gmelin Handbook: Cu: MVol.B4, 141, page 1757 - 1760
[14]Oriental Journal of Chemistry,2015,vol. 31,p. 1195 - 1200
- 5
-
7,8-dihydro-5,10,15,20-tetraphenylporphyrin
[ No CAS ]

-
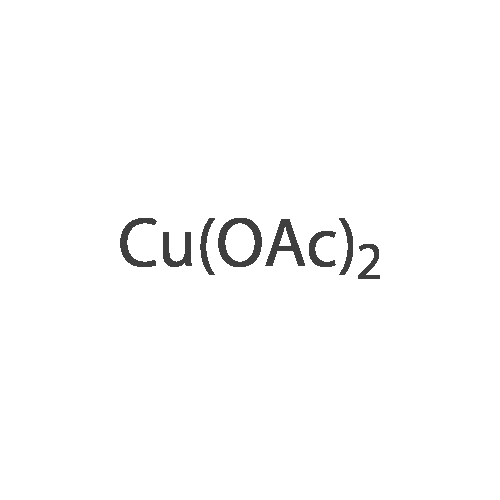 [ 142-71-2 ]
[ 142-71-2 ]

-
 [ 14172-91-9 ]
[ 14172-91-9 ]
- 6
-
 [ 10170-99-7 ]
[ 10170-99-7 ]

-
 [ 917-23-7 ]
[ 917-23-7 ]

-
 [ 14172-91-9 ]
[ 14172-91-9 ]
- 7
-
 [ 917-23-7 ]
[ 917-23-7 ]

-
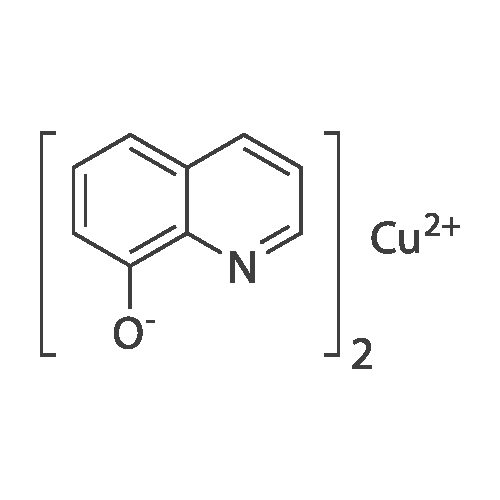 [ 13014-03-4 ]
[ 13014-03-4 ]

-
 [ 14172-91-9 ]
[ 14172-91-9 ]
- 8
-
 [ 917-23-7 ]
[ 917-23-7 ]

-
 [ 45227-32-5 ]
[ 45227-32-5 ]

-
 [ 14172-91-9 ]
[ 14172-91-9 ]
- 9
-
 [ 917-23-7 ]
[ 917-23-7 ]

-
 [ 7787-70-4 ]
[ 7787-70-4 ]

-
 [ 14172-91-9 ]
[ 14172-91-9 ]
- 10
-
 [ 917-23-7 ]
[ 917-23-7 ]

-
copper(II) oxide
[ No CAS ]

-
 [ 14172-91-9 ]
[ 14172-91-9 ]
- 11
-
 [ 917-23-7 ]
[ 917-23-7 ]

-
 [ 12775-96-1 ]
[ 12775-96-1 ]

-
 [ 14172-91-9 ]
[ 14172-91-9 ]
Reference:
[1]Journal of Chemical Research - Part S,
Journal of Chemical Research, Synopses,1981,p. 14 - 14
[2]Doklady Physical Chemistry,1983,vol. 271,p. 510 - 512
Dokl. Phys. Chem. (Transl. of Dokl. Akad. Nauk.),1983,vol. 271,p. 650 - 652
- 12
-
 [ 917-23-7 ]
[ 917-23-7 ]

-
 [ 377741-30-5 ]
[ 377741-30-5 ]

-
 [ 14172-91-9 ]
[ 14172-91-9 ]
- 13
-
 [ 917-23-7 ]
[ 917-23-7 ]

-
 [ 503276-25-3 ]
[ 503276-25-3 ]

-
 [ 14172-91-9 ]
[ 14172-91-9 ]
- 14
-
 [ 917-23-7 ]
[ 917-23-7 ]

-
copper(II) nitrate
[ No CAS ]

-
 [ 14172-91-9 ]
[ 14172-91-9 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With sodium carbonate; In water; at 350.0℃; for 0.0666667h;Inert atmosphere; Sealed tube; |
Using a 50 mL volumetric flask, 1.208 g of copper sulfate as a metal salt was dissolved in 50 mL of distilled water to prepare a 0.1 mol / L CuSO 4 aqueous solution. Next, 0.02 g (3.3 × 10 -5 mol) of tetraphenylporphyrin (TPP) as a compound having a porphyrin-type skeleton, 0.02 g (3.3 × 10 -5 mol) of copper sulfate Aqueous solution of sodium carbonate and 0.032 g of sodium carbonate equivalent to copper sulfate to prevent corrosion of the reaction vessel, and the interior of the reaction vessel was purged with argon and sealed. Next, the reaction vessel was charged into the sand bath set at 350 C. The reaction temperature in the reaction vessel reached the reaction temperature in about 4 minutes. |
- 15
-
 [ 917-23-7 ]
[ 917-23-7 ]

-
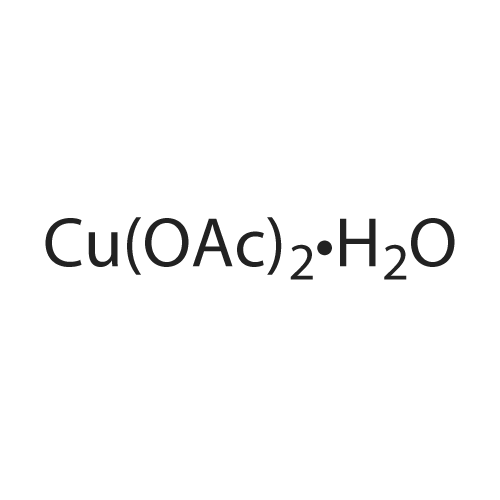 [ 6046-93-1 ]
[ 6046-93-1 ]

-
 [ 14172-91-9 ]
[ 14172-91-9 ]
- 16
-
 [ 917-23-7 ]
[ 917-23-7 ]

-
copper(II) perchlorate
[ No CAS ]

-
 [ 14172-91-9 ]
[ 14172-91-9 ]
- 17
-
 [ 917-23-7 ]
[ 917-23-7 ]

-
copper(II) ethylenediaminetetraacetate
[ No CAS ]

-
 [ 14172-91-9 ]
[ 14172-91-9 ]
- 18
-
 [ 917-23-7 ]
[ 917-23-7 ]

-
 [ 15416-63-4 ]
[ 15416-63-4 ]

-
 [ 14172-91-9 ]
[ 14172-91-9 ]

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping