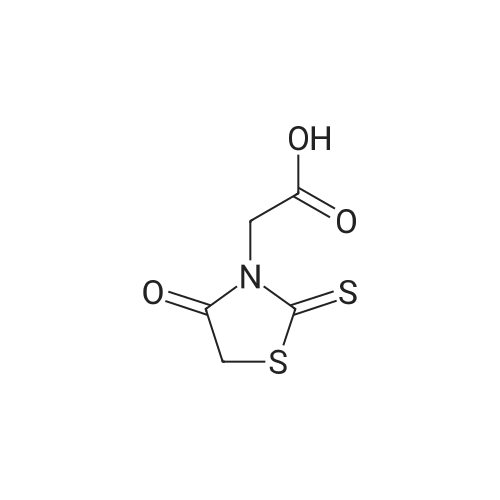
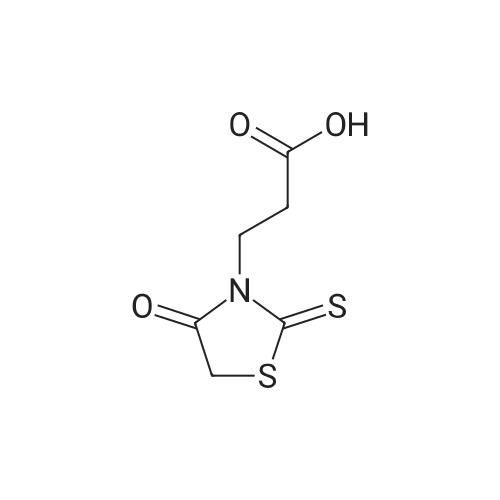
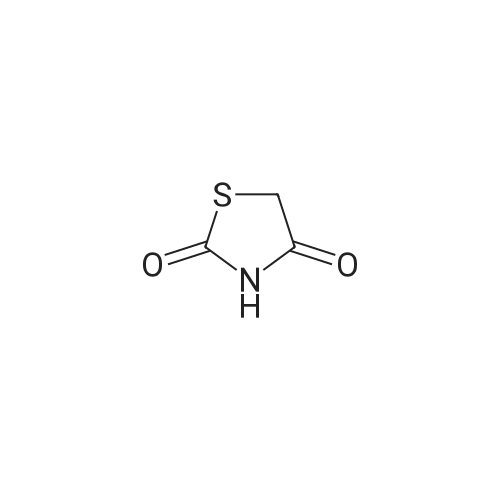
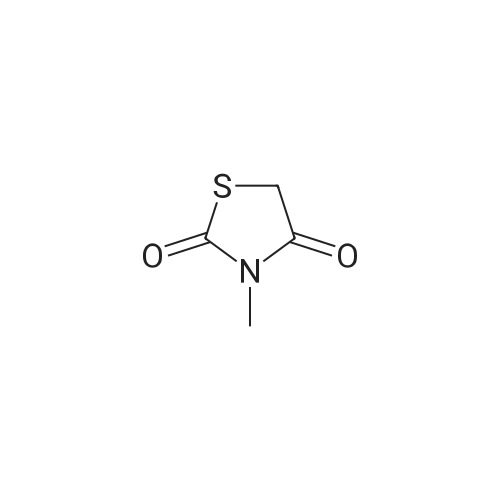
Alternatived Products of [ 141-84-4 ]
Product Details of [ 141-84-4 ]
| CAS No. : | 141-84-4 |
MDL No. : | MFCD00005488 |
| Formula : |
C3H3NOS2
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | KIWUVOGUEXMXSV-UHFFFAOYSA-N |
| M.W : |
133.19
|
Pubchem ID : | 1201546 |
| Synonyms : |
|
Application In Synthesis of [ 141-84-4 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 141-84-4 ]
- 1
-
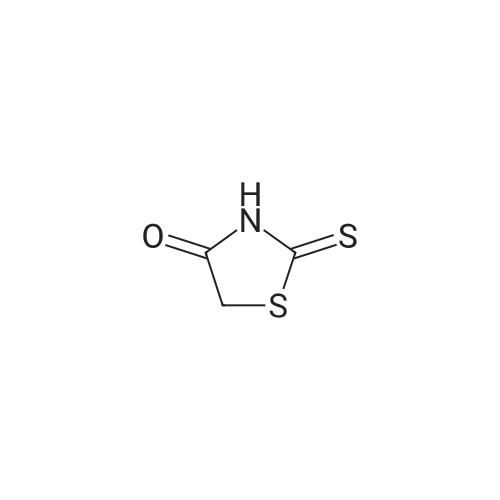 [ 141-84-4 ]
[ 141-84-4 ]

-
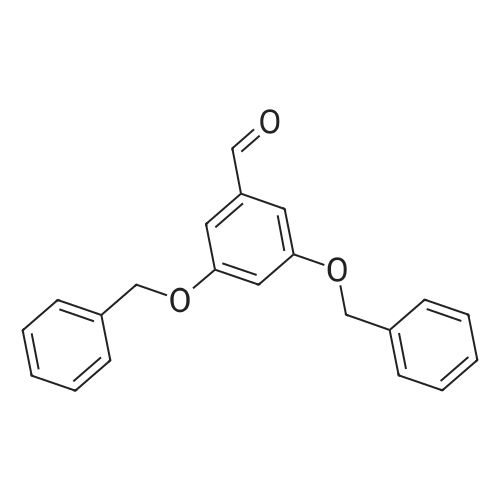 [ 14615-72-6 ]
[ 14615-72-6 ]

-
C24H19NO3S2
[ No CAS ]
- 2
-
 [ 141-84-4 ]
[ 141-84-4 ]

-
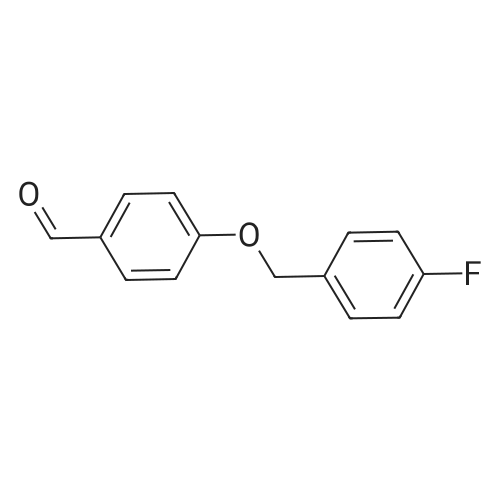 [ 56442-17-2 ]
[ 56442-17-2 ]

-
 [ 247566-73-0 ]
[ 247566-73-0 ]
| Yield | Reaction Conditions | Operation in experiment |
| 91.15% |
With sodium acetate; In acetic acid; for 7h;Reflux; |
To a stirred solution of 4-(4-Fluoro-benzyloxy)-benzaldehyde (250 mg, 1.09 mmol) , rhodanine (145 mg., 1.09 mmol) in acetic acid (10 ml) was added sodium acetate (357 mg, 4.36 mmol) and heated under reflux for 7 h . After cooling to room temperature, water (15ml) was added and solid precipitate was filtered ,washed with water (10ml) and hexane(10 ml) and dried under vacuum to afford a yellow solid product (340 mg , 91.15%). |
- 3
-
 [ 141-84-4 ]
[ 141-84-4 ]

-
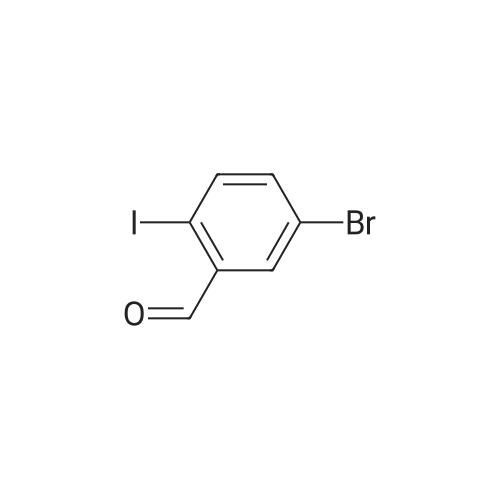 [ 689291-89-2 ]
[ 689291-89-2 ]

-
5-(5-bromo-2-iodobenzylidene)-2-thioxothiazolidin-4-one
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 81% |
With sodium hydroxide; In ethanol;Reflux; |
General procedure: The 2-thioxo-4-thiazolidinone (1mmol), benzaldehydes (1 mmol) and NaOH (1.0 mmol) were added to ethanol withtotal volume of 15 mL. The reaction mixture was heated to reflux and stirredfor 2-24 h. After cooling to room temperature, the mixture was concentrated under reduced pressure, neutralized to pH 7.0 with dilute hydrochloric, and then extracted with ethyl acetate (3×100 mL). The combined organic extracts were washed with brine, dried over anhydrous Na2SO4, and concentrated. The resulting residue was recrystallization from ethanol. |
- 4
-
 [ 141-84-4 ]
[ 141-84-4 ]

-
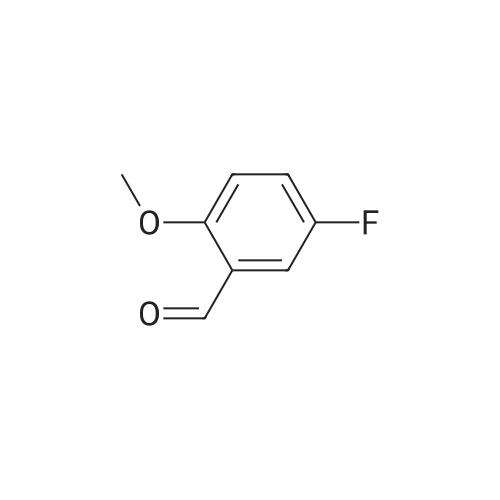 [ 19415-51-1 ]
[ 19415-51-1 ]

-
5-(5-fluoro-2-methoxybenzylidene)-2-thioxothiazolidin-4-one
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 82% |
With sodium hydroxide; In ethanol;Reflux; |
General procedure: The 2-thioxo-4-thiazolidinone (1mmol), benzaldehydes (1 mmol) and NaOH (1.0 mmol) were added to ethanol withtotal volume of 15 mL. The reaction mixture was heated to reflux and stirredfor 2-24 h. After cooling to room temperature, the mixture was concentrated under reduced pressure, neutralized to pH 7.0 with dilute hydrochloric, and then extracted with ethyl acetate (3×100 mL). The combined organic extracts were washed with brine, dried over anhydrous Na2SO4, and concentrated. The resulting residue was recrystallization from ethanol. |
- 5
-
 [ 141-84-4 ]
[ 141-84-4 ]

-
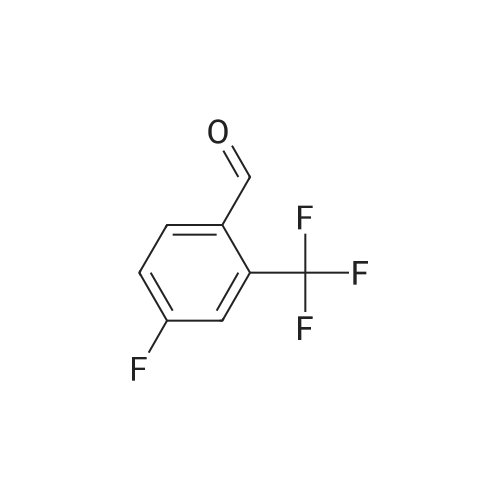 [ 90176-80-0 ]
[ 90176-80-0 ]

-
5-(4-fluoro-2-(trifluoromethyl)benzylidene)-2-thioxothiazolidin-4-one
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 81% |
With sodium hydroxide; In ethanol;Reflux; |
General procedure: The 2-thioxo-4-thiazolidinone (1mmol), benzaldehydes (1 mmol) and NaOH (1.0 mmol) were added to ethanol withtotal volume of 15 mL. The reaction mixture was heated to reflux and stirredfor 2-24 h. After cooling to room temperature, the mixture was concentrated under reduced pressure, neutralized to pH 7.0 with dilute hydrochloric, and then extracted with ethyl acetate (3×100 mL). The combined organic extracts were washed with brine, dried over anhydrous Na2SO4, and concentrated. The resulting residue was recrystallization from ethanol. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping