Alternatived Products of [ 13937-08-1 ]
Product Details of [ 13937-08-1 ]
| CAS No. : | 13937-08-1 |
MDL No. : | MFCD04038932 |
| Formula : |
C7H12O5
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | VXZQTXSCMRPKMH-UHFFFAOYSA-N |
| M.W : |
176.17
|
Pubchem ID : | 139648 |
| Synonyms : |
|
Application In Synthesis of [ 13937-08-1 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 13937-08-1 ]
- 1
-
 [ 74-89-5 ]
[ 74-89-5 ]

-
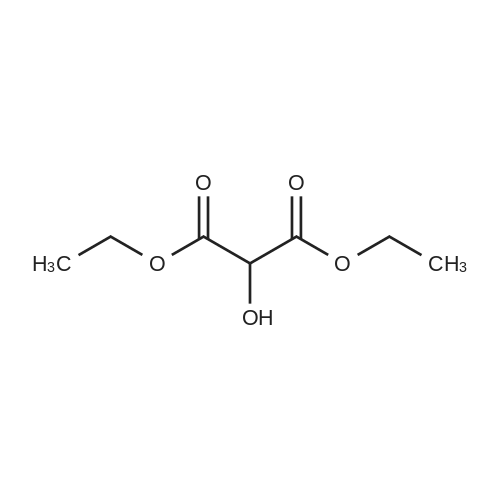 [ 13937-08-1 ]
[ 13937-08-1 ]

-
hydroxy-malonic acid bis-methylamide
[ No CAS ]
- 2
-
 [ 51-64-9 ]
[ 51-64-9 ]

-
 [ 13937-08-1 ]
[ 13937-08-1 ]

-
 [ 112350-94-4 ]
[ 112350-94-4 ]
- 3
-
 [ 13937-08-1 ]
[ 13937-08-1 ]

-
2-hydroxymalonamide
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 97.5% |
With ammonium hydroxide; at 90℃; for 3h; |
176g of 2-hydroxymalonic acid diethyl ester (1mol) and 250g, a concentration of 28wt% aqueous ammonia (2mol) mixed, and then heated to 90 , the reaction 3h.After the reaction was completed, water was added and the mixture was extracted three times with ethyl acetate. The organic layer was spin-dried,115 g of 2-hydroxymalonamide were obtained in a yield of 97.5%. |
- 4
-
 [ 13937-08-1 ]
[ 13937-08-1 ]

-
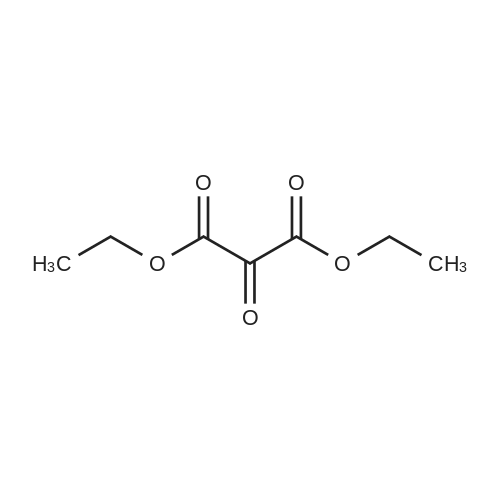 [ 609-09-6 ]
[ 609-09-6 ]
- 5
-
 [ 545-26-6 ]
[ 545-26-6 ]

-
 [ 13937-08-1 ]
[ 13937-08-1 ]
- 6
-
 [ 609-09-6 ]
[ 609-09-6 ]

-
 [ 13937-08-1 ]
[ 13937-08-1 ]
Reference:
[1]Bulletin de la Societe Chimique de France,1937,vol. <5> 4,p. 31,41
[2]Journal of the University of Bombay, Science: Physical Sciences, Mathematics, Biological Sciences and Medicine,1932,vol. 1/,p. 49
Chemisches Zentralblatt,1933,vol. 104,p. 946
[3]Chemical Communications,2006,p. 1218 - 1220
[4]Chemical Communications,2013,vol. 49,p. 1184 - 1186
- 7
-
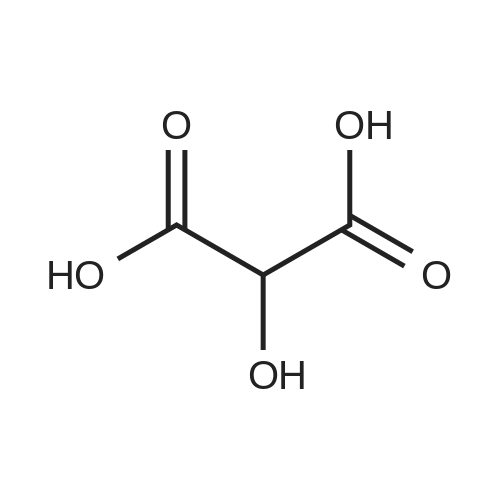 [ 80-69-3 ]
[ 80-69-3 ]

-
 [ 64-17-5 ]
[ 64-17-5 ]

-
 [ 13937-08-1 ]
[ 13937-08-1 ]
- 8
-
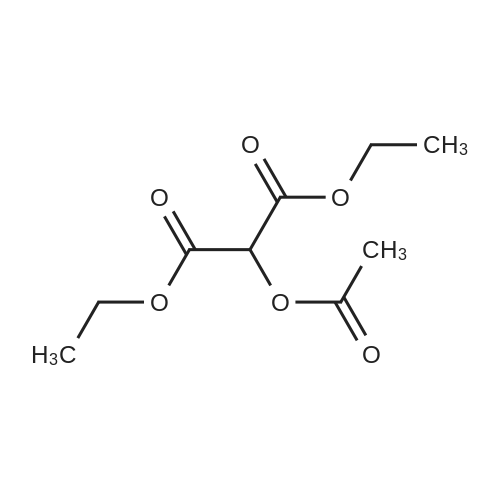 [ 5468-23-5 ]
[ 5468-23-5 ]

-
 [ 13937-08-1 ]
[ 13937-08-1 ]
- 9
-
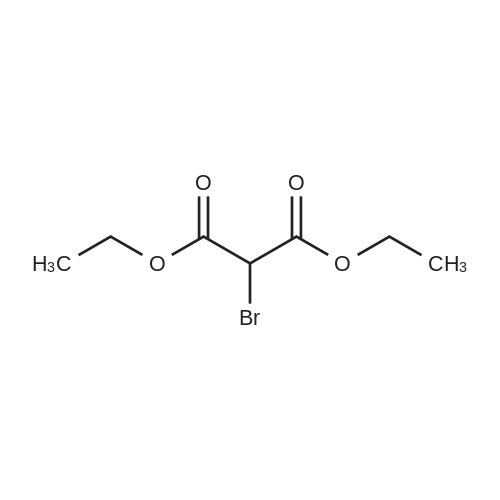 [ 685-87-0 ]
[ 685-87-0 ]

-
 [ 13937-08-1 ]
[ 13937-08-1 ]
- 10
-
 [ 109-92-2 ]
[ 109-92-2 ]

-
 [ 13937-08-1 ]
[ 13937-08-1 ]

-
 [ 128302-74-9 ]
[ 128302-74-9 ]
- 11
-
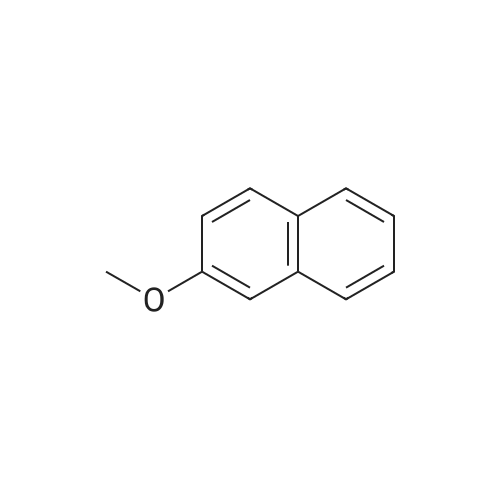 [ 93-04-9 ]
[ 93-04-9 ]

-
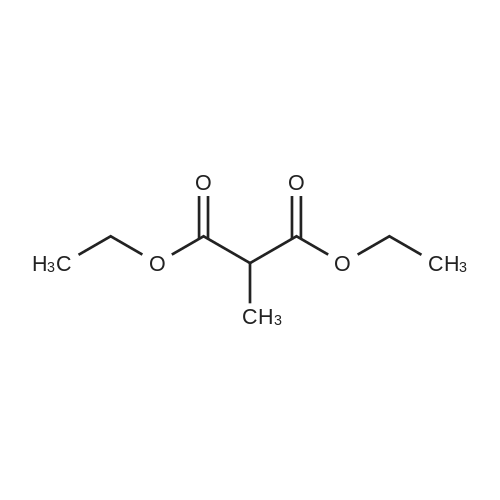 [ 609-08-5 ]
[ 609-08-5 ]

-
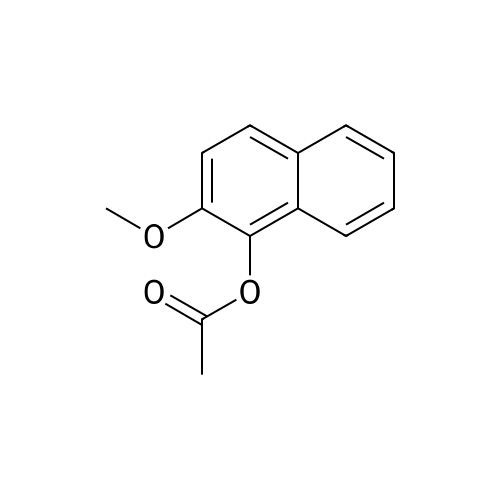 [ 27581-07-3 ]
[ 27581-07-3 ]

-
 [ 118647-58-8 ]
[ 118647-58-8 ]

-
 [ 118647-59-9 ]
[ 118647-59-9 ]

-
 [ 13937-08-1 ]
[ 13937-08-1 ]
- 12
-
 [ 105-53-3 ]
[ 105-53-3 ]

-
 [ 13937-08-1 ]
[ 13937-08-1 ]
| Yield | Reaction Conditions | Operation in experiment |
| 96.6% |
With 3,3-dimethyldioxirane; nickel diacetate; In ethanol; for 5h;Reflux; |
160g of diethyl malonate (1mol) was dissolved in 300mL of ethanol,Add 111g ofDimethyldioxirane (1.5 mol) and 0.5 g of nickel acetate,Then heated to reflux, the reaction 5h.After the reaction, the ethanol is evaporated to dryness, water is added,Extract with ethyl acetate three times and spin the organic layer to 170 gDiethyl 2-hydroxymalonate was obtained in a yield of 96.6%. |
| 33% |
With Oxone; In aq. phosphate buffer; water; ethyl acetate; acetone; at 25℃;pH 8.0; |
General procedure: To a round-bottomed flask containing alkene 118(1.0 mmol), ethyl acetate (10 mL), acetone (5.0 mL) andphosphate buffer (1.0 mol L-1 K2HPO4/KH2PO4, pH 8,10 mL), under magnetic stirring, was added a solution ofOxone (1.3 mmol for 1a or 1.5 mmol for 1b) in water(5.0 mL) over a period of 1 h. The reaction mixture wasstirred until TLC (hexane/EtOAc, 80:20, v/v) showedcompletion of the reaction (see below). The insolublesolid was separated by filtration under reduced pressureand washed with EtOAc. The filtrate was washed with1.0 mol L-1 Na2S2O3 and brine, dried over anhydrousNa2SO4, and concentrated under reduced pressure to givethe epoxides as pale yellow oil. The crude mixture waspurified by silica gel chromatography (hexane:EtOAc,80:20, v/v) to provide epoxides 2a and 2b. |
- 13
-
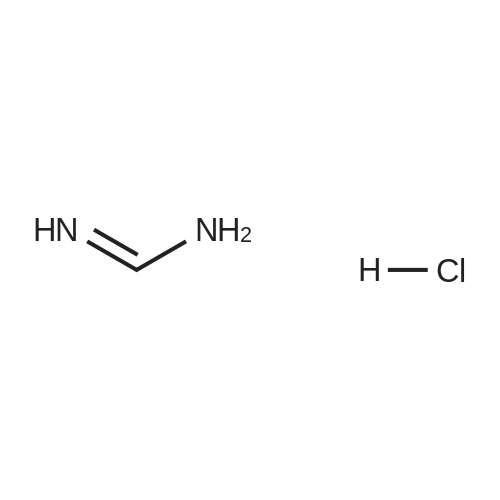 [ 6313-33-3 ]
[ 6313-33-3 ]

-
 [ 13937-08-1 ]
[ 13937-08-1 ]

-
4,5,6-trihydroxypyrimidine
[ No CAS ]
- 14
-
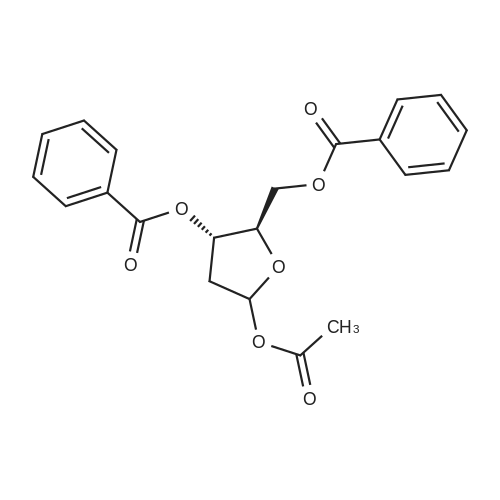 [ 51255-12-0 ]
[ 51255-12-0 ]

-
 [ 13937-08-1 ]
[ 13937-08-1 ]

-
3,5-bis-O-benzoyl-2-deoxy-1-O-(2-diethylmalonyl)-β-D-erythro-pentofuranose
[ No CAS ]

-
3,5-bis-O-benzoyl-2-deoxy-1-O-(2-diethylmalonyl)-α-D-erythro-pentofuranose
[ No CAS ]
- 15
-
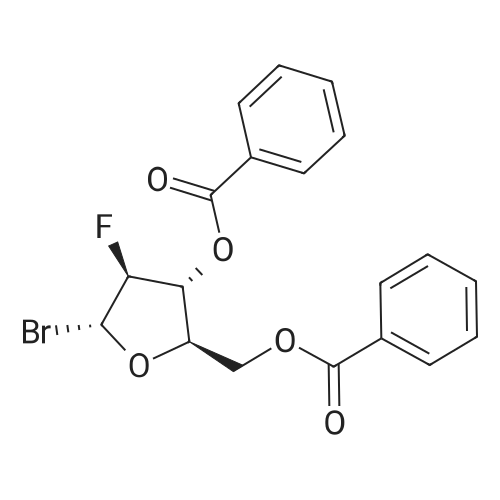 [ 97614-44-3 ]
[ 97614-44-3 ]

-
 [ 13937-08-1 ]
[ 13937-08-1 ]

-
3,5-bis-O-benzoyl-2-deoxy-2-fluoro-α-D-arabinofuranose
[ No CAS ]

-
3,5-bis-O-benzoyl-2-deoxy-1-O-(2-diethylmalonyl)-2-fluoro-α-D-arabino-pentofuranose
[ No CAS ]
- 16
-
 [ 508210-05-7 ]
[ 508210-05-7 ]

-
 [ 13937-08-1 ]
[ 13937-08-1 ]

-
 [ 183550-77-8 ]
[ 183550-77-8 ]
| Yield | Reaction Conditions | Operation in experiment |
|
|
EXAMPLE 1 1,800 ml of absolute ethanol is saturated with HCl gas at room temperature and then, at a maximum of 30 C., first 537 g (trimethylsilyloxy)tricyanomethane are added dropwise and, after stirring for 30 minutes, 54 g of water are added dropwise. After heating at the reflux temperature for 1 hour, followed by cooling, the NH4 Cl is filtered off with suction and the filtrate is concentrated in vacuo. The liquid residue is distilled in vacuo (over a 1 meter long Vigreux column). 316 g (55% of theory) of diethyl tartronate of boiling point 111-113 C./16 mbar are obtained. |
|
|
EXAMPLE 2 300 ml of anhydrous ethanol are saturated with HCl gas at 10 C. and then, at 0 C., 102 g of (trimethylsilyloxy)tricyanomethane are added. The mixture is then stirred at 15 C. for 1 hour and subsequently diluted with 300 ml of water. After stirring for 30 minutes, ice-water is added and the mixture is extracted with methylene chloride. Working up the methylene chloride extract and vacuum distillation of the residue provides 86 g (50% of theory) of diethyl tartronate of boiling point 112-113 C./16 mbar. |
- 19
-
 [ 64-17-5 ]
[ 64-17-5 ]

-
tartronate barium
[ No CAS ]

-
 [ 13937-08-1 ]
[ 13937-08-1 ]

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping