| 70% |
|
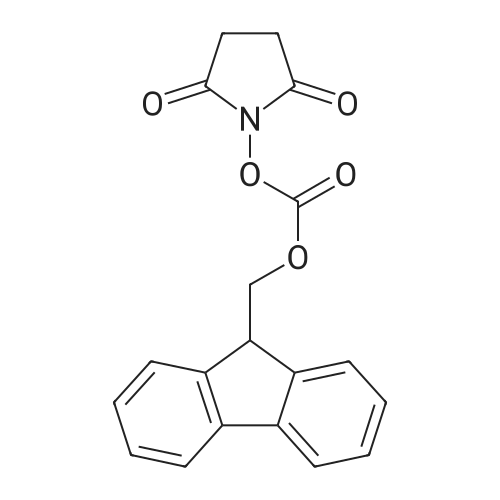
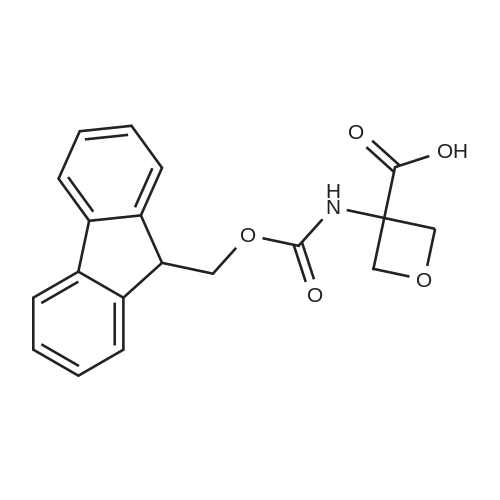
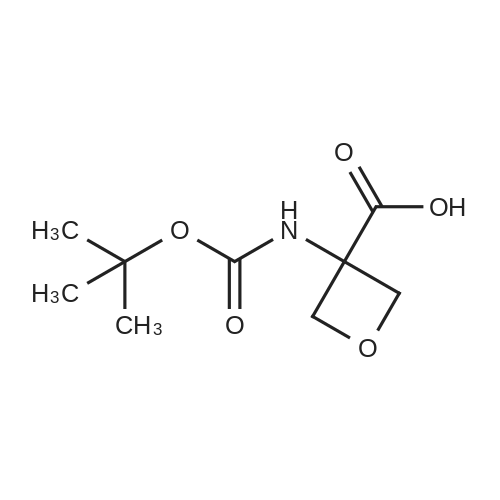
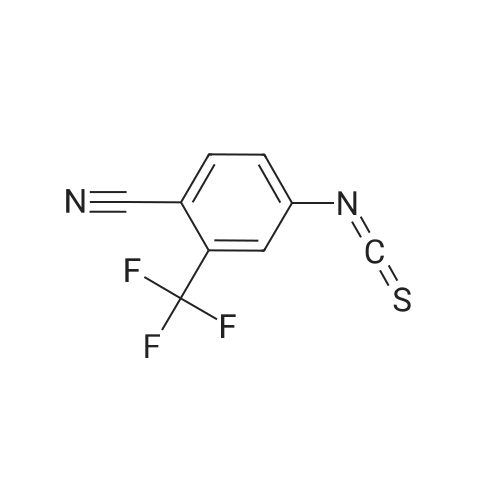
The mixture of compound 40(500 mg, 1.22 mmol), 3-minooxetane-3-carboxylic acid (57)(215 mg, 1.84 mmol) [38], K2CO3 (590 mg, 4.27 mmol), CuI (47 mg,0.24 mmol) and 1 drop of Et3N dissolved in DMF (4 mL) and water(1 mL) was stirred for 15 min, then 2-acetylcyclohexanone (190 mg,1.35 mmol) was added and the mixture was stirred for 15 h at100. After cooling the mixture was rotovapped and subjected toHPLC with neutral mobile phase to afford 340 mg (70%) of 3-(3-fluoro-4-{methyl-[2-(trimethylsilyl)ethoxymethyl]carbamoyl}phenylamino)oxetane-3-carboxylic acid (58). MS (ESI) [MH]399. 1H NMR (400 MHz, DMSO-d6) d 7.61 (m, 1H), 7.53 (t, J 8.8 Hz,1H), 6.21 (d, J 8.4 Hz, 1H), 5.96 (d, J 13.6 Hz, 1H), 4.97 (d,J 6.0 Hz, 2H), 4.55 (d, J 6.0 Hz, 2), 2.73 (d, J 4.4 Hz, 3), 2.21(m, 2), 1.12 (m, 2), 0.14 (s, 9). The mixture of compound 40(500 mg, 1.22 mmol), 3-minooxetane-3-carboxylic acid (57)(215 mg, 1.84 mmol),38 K2CO3 (590 mg, 4.27 mmol), CuI (47 mg,0.24 mmol) and 1 drop of Et3N dissolved in DMF (4 mL) and water(1 mL) was stirred for 15 min, then 2-acetylcyclohexanone (190 mg,1.35 mmol) was added and the mixture was stirred for 15 h at100. After cooling the mixture was acidified with hydrochloricacid to pH 3 and extracted with ethyl acetate. The organic layerwas washed with cold water, with brine, dried over Na2SO4 and thesolvent evaporated under reduced pressure to give 287 mg (88%) of3-[3-fluoro-4-(methylcarbamoyl)phenylamino]oxetane-3-carboxylic acid (59). MS (ESI) [MH] 269. 1H NMR (400 MHz,DMSO-d6), d 7.69 (m, 1H), 7.48 (t, J 8.8 Hz, 1H), 6.15 (d, J 8.4 Hz,1H), 5.96 (d, J 13.6 Hz, 1H), 4.97 (d, J 6.0 Hz, 2H), 4.55 (d,J 6.0 Hz, 2), 2.73 (d, J 4.4 Hz, 3). Sodium hydride (66 mg,1.65 mmol, 60% in oil) was added at 0 to the solution of compound58 (600 mg, 1.5 mmol) in DMF (6 mL). The resulting mixturewas stirred for 0.5 h keeping same temperature. After the reactionwas completed, iodomethane (0.14 mL, 2.25 mmol) was added tothe mixture and stirring was continued for 6 h at 0. Finally, thesolution was accurately poured into cold water, extracted withethyl acetate, dried over Na2SO4 and rotovapped. The obtainedcrude methyl 3-(3-fluoro-4-{methyl-[2-(trimethylsilyl)ethoxymethyl]carbamoyl}phenylamino)oxetane-3-carboxylate (60)was subjected to HPLC with mobile phase containing 0.1% of TFA toafford 46 mg (11%) of methyl 3-[3-fluoro-4-(methylcarbamoyl)phenylamino]oxetane-3-carboxylate (61). MS (ESI) [MH] 283.1H NMR (400 MHz, CDCl3) d 7.96 (t, J 9.2 Hz, 1), 6.60 (brs, 1),6.31 (dd, J1 8.8 Hz, J2 2.0 Hz, 1), 6.12 (dd, J1 14.4 Hz,J2 2.0 Hz, 1), 5.13 (d, J 6.4 Hz, 2), 5.03 (brs, 1), 4.76 (d,J 6.4 Hz, 2), 3.84 (s, 3), 3.01 (d, J 4.4 Hz, 3). Compound 42(63 mg, 0.276 mmol) was added to the solution of compound 59 or61 (0.25 mmol) in DMF (1 mL). The resulting mixturewas stirred for12 h at 80 under argon atmosphere. After the reaction wascompleted, the solutionwas poured into cold water, extracted withethyl acetate, dried over Na2SO4 and rotovapped. Separation byHPLC afforded 17 mg (16%) of N-[4-cyano-3-(trifluoromethyl)phenyl]-3-[3-fluoro-4-(methylcarbamoyl)phenylamino]oxetane-3-carboxamide (62).MS (ESI) [MH] 437. 1H NMR (400 MHz, DMSOd6)d 10.59 (s, 1), 8.30 (s, 1), 8.16 (d, J 8.8 Hz, 1), 8.08 (d,J 8.4 Hz, 1), 7.73 (m, 1), 7.55 (s, 1), 7.49 (t, J 8.4 Hz, 1), 6.23(d, J 10.0 Hz, 1), 6.10 (d, J 13.6 Hz, 1), 5.09 (d, J 6.8 Hz, 2),4.59 (d, J 6.4 Hz, 2), 2.70 (d, J 4.0 Hz, 3). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping