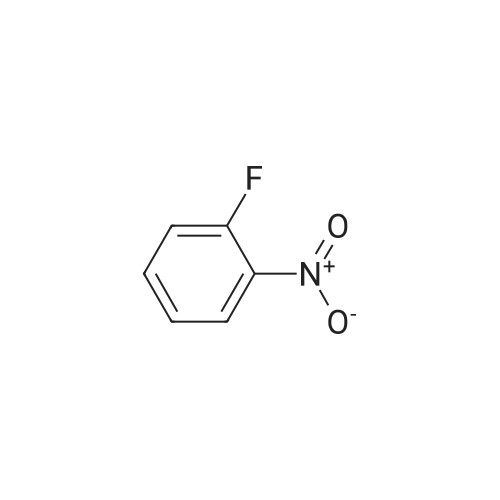
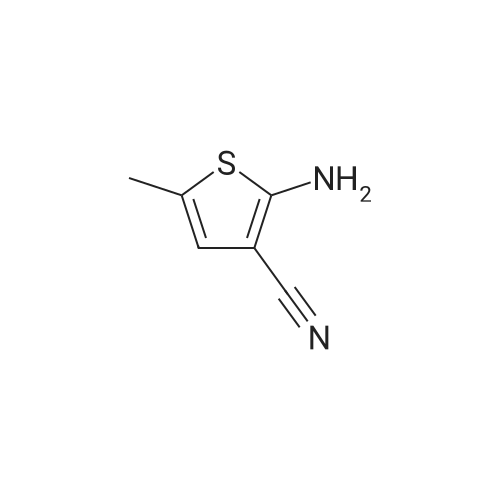
| 60% |
With potassium hydroxide;N-benzyl-N,N,N-triethylammonium chloride; In DMF (N,N-dimethyl-formamide); at 0 - 25℃; for 5h; |
To a stirred mixture of potassium hydroxide (59.1 g), benzyltriethylammonium chloride (1.2 g) and N,N-dimethylformamide (70 mL) in a 250 mL flask was added dropwise a solution of 2-amino-5-methylthiophene-3- carbonitrile (73 g) and 1-fluoro-2-nitrobenzene (74.5 g) in N,N dimethylformamide (175 mL) while maintaining the temperature between at 20-25 °C with an ice/salt bath. After the addition was complete, the mixture was stirred between 20-25°C for 5 hours, then poured onto ice/water (400 mL), and extracted with dichloromethane (480 mL). The organic layer was separated, and the aqueous layer was extracted twice with dichloromethane (240 mLx2). Water (400 mL) was added to the combined organic extracts, and the pH was adjusted between 8-9 with 2N hydrochloric acid. The organic layer was separated and washed with water (400 mL). The solvent was removed under reduced pressure to afford a residue that was crystallized from ethanol (300 mL) to give 2- (2-nitroanilino)-5-methylthiophene-3- carbonitrile (82.2 g). Yield: 60percent |
|
With potassium hydroxide; In acetonitrile; at 0 - 5℃; for 3h; |
Potassium hydroxide (101.4 g) in acetonitrile (150 ml) is taken under nitrogen and cooled to 0-5°C. A solution of <strong>[138564-58-6]2-amino-5-methylthiophene-3-carbonitrile</strong> (100 g) and o-fluoronitrobenzene (122.6 g) in acetonitrile (550 ml) is added. The reaction is then stirred for 3 hours and chilled water is added. The solid thus obtained is filtered off and air-dried. The solid is crystallized from water-methanol mixture and the crystallized solid is dried under vacuum at 40-45°C to obtain the title compound (140 g). |
|
In tetrahydrofuran; |
2. 2-(2-Nitroanilino)-5-methylthiophene-3-carbonitrile To a stirred slurry of sodium hydride (14.4 g, 50percent dispersion in oil, 0.3 mol) in dry tetrahydrofuran (50 mL) under nitrogen was added, dropwise, a solution of 2-fluoronitrobenzene (28.2 g, 0.2 mol) and 2-amino-5-methylthiophene3-carbonitrile (27.6 g, 0.2 mol) in dry tetrahydrofuran (250 mL). The mixture was stirred at 25° C. for 24 hours, poured onto cracked ice and extracted into dichloromethane (3*500 mL). The combined extracts were washed with 2N hydrochloric acid (2*200 mL), water (2*200 mL), dried over magnesium sulphate and the solvent removed under reduced pressure. The residue was crystallized from ethanol to give the title compound, (35.2 g), m.p. 99°-102° C. |
|
In tetrahydrofuran; |
2. 2-(2-Nitroanilino)-5-methylthiophene-3-carbonitrile To a stirred slurry of sodium hydride (14.4 g, 50percent dispersion in oil, 0.3 mol) in dry tetrahydrofuran (50 mL) under nitrogen was added, dropwise, a solution of 2-fluoro-nitrobenzene (28.2 g, 0.2 mol) and <strong>[138564-58-6]2-amino-5-methylthiophene-3-carbonitrile</strong> (27.6 g, 0.2 mol) in dry tetrahydrofuran (250 mL). The mixture was stirred at 25° C. for 24 hours, poured onto cracked ice and extracted into dichloromethane (3*500 mL). The combined extracts were washed with 2N hydrochloric acid (2*200 mL), water (2*200 mL), dried over magnesium sulfate and the solvent removed under reduced pressure. The residue was crystallized from ethanol to give the title compound, (35.2 g), m.p. 99°-102° C. |
|
In tetrahydrofuran; |
2. 2-(2-Nitroanilino)-5-methylthiophene-3-carbonitrile To a stirred slurry of sodium hydride (14.4 g, 50percent dispersion in oil, 0.3 mol) in dry tetrahydrofuran (50 mL) under nitrogen was added, dropwise, a solution of 2-fluoro-nitrobenzene (28.2 g, 0.2 mol) and <strong>[138564-58-6]2-amino-5-methylthiophene-3-carbonitrile</strong> (27.6 g, 0.2 mol) in dry tetrahydrofuran (250 mL). The mixture was stirred at 25° C. for 24 hours, poured onto cracked ice and extracted into dichloromethane (3*500 mL). The combined extracts were washed with 2N hydrochloric acid (2*200 mL) water (2*200 mL), dried over magnesium sulfate and the solvent removed under reduced pressure. The residue was crystallized from ethanol to give the title compound, (35.2 g), m.p. 99°-102° C. |
|
In tetrahydrofuran; |
2. (2-(2-Nitroanilino)-5-methylthiophene-3-carbonitrile To a stirred slurry of sodium hydride (14.4 g, 50percent dispersion in oil, 0.3 mol) in dry tetrahydrofuran (50 mL) under nitrogen was added, dropwise, a solution of 2-fluoro-nitrobenzene (28.2 g, 0.2 mol) and <strong>[138564-58-6]2-amino-5-methylthiophene-3-carbonitrile</strong> (27.6 g, 0.2 mol) in dry tetrahydrofuran (250 mL). The mixture was stirred at 25° C. for 24 hours, poured onto cracked ice and extracted into dichloromethane (3*500 mL). The combined extracts were washed with 2N hydrochloric acid (2*200 mL), water (2*200 mL), dried over magnesium sulfate and the solvent removed under reduced pressure. The residue was crystallized from ethanol to give the title compound, (35.2 g), m.p. 99°-102° C. |
|
In tetrahydrofuran; |
2. 2-(2-Nitroanilino)-5-methylthiophene-3-carbonitrile To a stirred slurry of sodium hydride (14.4 g, 50percent dispersion in oil, 0.3 mol) in dry tetrahydrofuran (50 mL) under nitrogen was added, dropwise, a solution of 2-fluoro-nitrobenzene (28.2 g, 0.2 mol) and 2-amino-5- methylthiophene-3-carbonitrile (27.6 g, 0.2 mol) in dry tetrahydrofuran (250 mL). The mixture was stirred at 25° C. for 24 hours, poured onto cracked ice and extracted into dichloromethane (3*500 mL). The combined extracts were washed with 2N hydrochloric acid (2 *200 mL), water (2*200 mL), dried over magnesium sulfate and the solvent removed under reduced pressure. The residue was crystallized from ethanol to give the title compound, (35.2 g), m.p. 99°-102° C. |
|
With potassium hydroxide; In isopropyl alcohol; |
2-fIuoronitrobenzene is condensed with 5-Amino-4-Cyano-2-Methyl Thiophene in Isopropyl alcohol and Potassium Hydroxide powder give 4-cyano-2-methyl-1-(2-nitrophenyl amino) Thiophene |
|
With potassium hydroxide; In acetonitrile; at 0 - 5℃; for 3h; |
EXAMPLE 2; Preparation of 2-(2-Nitroanilino)-5-methylthiophene-3-carbonitrilePotassium hydroxide (101.4 g) in acetonitrile (150 ml) is taken under nitrogen and cooled to 0-5° C. A solution of <strong>[138564-58-6]2-amino-5-methylthiophene-3-carbonitrile</strong> (100 g) and o-fluoronitrobenzene (122.6 g) in acetonitrile (550 ml) is added. The reaction is then stirred for 3 hours and chilled water is added. The solid thus obtained is filtered off and air-dried. The solid is crystallized from water-methanol mixture and the crystallized solid is dried under vacuum at 40-45° C. to obtain the title compound (140 g). |
|
With potassium hydroxide; In acetonitrile; at 0 - 5℃;Inert atmosphere; |
Example 2; Preparation of 2-(2-nitroanilino)-5-methylthiophene-3-carbonitrilePotassium hydroxide (101 g) in acetonitrile (150 ml) was taken under nitrogen and cooled to 0-5° C. A solution of <strong>[138564-58-6]2-amino-5-methylthiophene-3-carbonitrile</strong> (100 g) and ortho-fluoronitrobenzene (122 g) in acetonitrile (550 ml) was added. The reaction mixture was then stirred for 3 hours and chilled water was added into the reaction mixture. The solid was separated out. The solid thus obtained was filtered off and air-dried. The solid was crystallized from water-methanol mixture and the crystallized solid was dried under vacuum at 40-45° C. to obtain the title compound (140 g). |
|
With potassium hydroxide; In dimethyl sulfoxide; |
Step E 5-Methyl-2-((2-nitrophenyl)amino)thiophene-3-carbonitrile To a solution of <strong>[138564-58-6]2-amino-5-methylthiophene-3-carbonitrile</strong> (13.8 g, 100 mmol) and 1-fluoro-2-nitrobenzene (16.92 g, 120 mmol) in dimethylsulfoxide was added potassium hydroxide (11.2 g, 200 mmol). The reaction mixture was stirred at room temperature overnight. The mixture was diluted with water, and the resulting suspension was filtered. The filtered cake was dried to give 5-methyl-2-((2-nitrophenyl)amino)thiophene-3-carbonitrile as a red solid used without further purification. 1H NMR: (400 MHz, CDCl3) delta 9.69 (s, 1H), 8.27-8.25 (m, 1H), 7.56-7.52 (m, 1H), 7.23-7.20 (m, 1H), 7.0-6.96 (m, 1H), 6.80 (s, 1H), 2.49 (s, 3H). |
|
With potassium hydroxide; In dimethyl sulfoxide; at 20℃; |
To a solution of <strong>[138564-58-6]2-amino-5-methylthiophene-3-carbonitrile</strong> (13.8 g, 100mmol) and 1-fluoro-2-nitrobenzene (16.92 g, 120 mmol) in dirnethylsulfoxide wasadded potassium hydroxide (11.2 g, 200 mmol). The reaction mixture was stirred atroom temperature overnight The mixture was diluted with water, and the resultingsuspension was filtered. The filtered cake was dried to give 5-methyl-2-((2-nitropllenyl)amino)thiophene-3-carbonitrile as a red solid used without furtherpurification. 1H NMR: (400 MHz, CDCb) o 9.69 (s, 1 H), 8.27-8.25 (m, 1 H), 7.56-7.52(m, 1 H), 7.23-7.20 (m, 1 H), 7.0-6.96 (m, 1 H), 6.80 (s, 1 H), 2.49(s, 3H). |
|
With potassium hydroxide; In dimethyl sulfoxide; at 20℃; |
To a solution of <strong>[138564-58-6]2-amino-5-methylthiophene-3-carbonitrile</strong> (13.8 g, 100 mmol) and 1-fluoro-2-nitrobenzene (16.92 g, 120 mmol) in dimethylsulfoxide was added potassium hydroxide (11.2 g, 200 mmol). The reaction mixture was stirred at room temperature overnight. The mixture was diluted with water, and the resulting suspension was filtered. The filtered cake was dried to give 5-methyl-2-((2-nitrophenyl)amino)thiophene-3-carbonitrile as a red solid used without further purification. 1H NMR (400 MHz, CDCl3) delta 9.69 (s, 1H), 8.27-8.25 (m, 1H), 7. 56-7.52 (m, 1H), 7.23-7.20 (m, 1H), 7.0-6 .96 (m,1H), 6. 80 (s, 1H) , 2 .49 (s, 3H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping