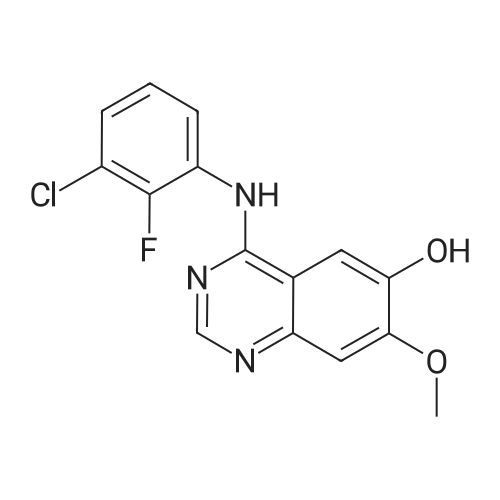
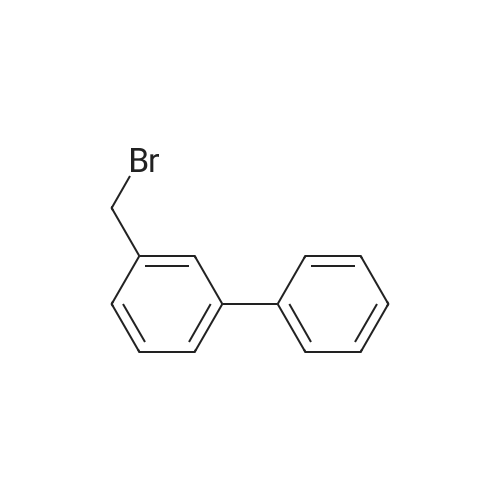
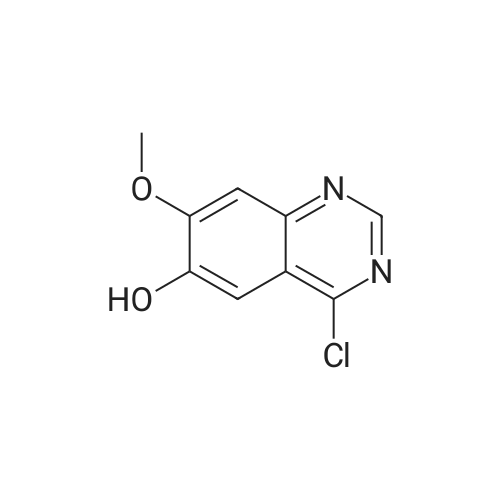
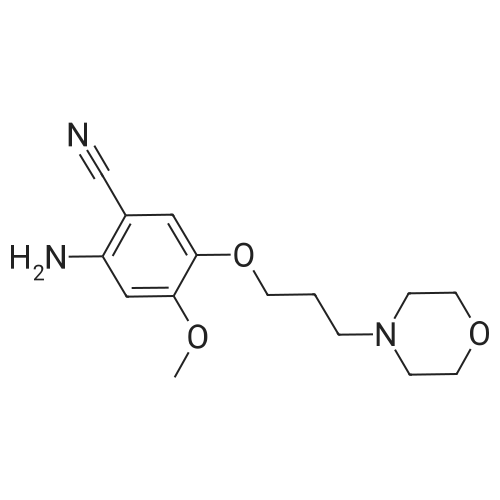
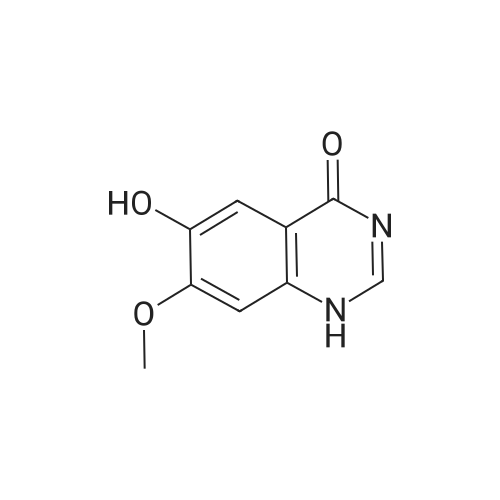
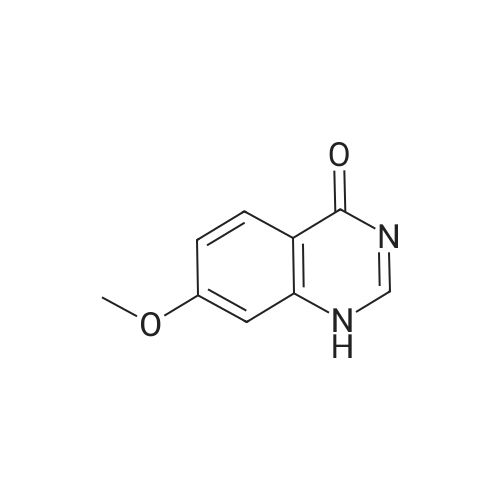
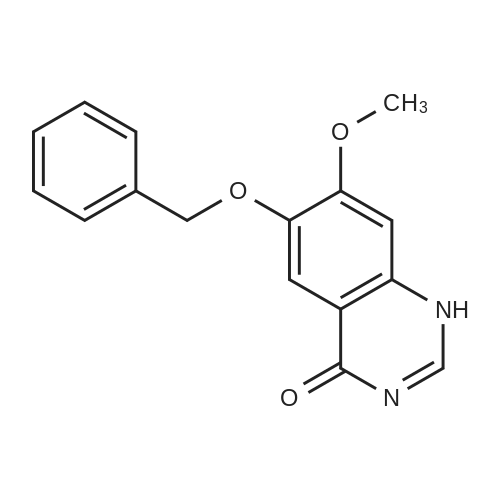
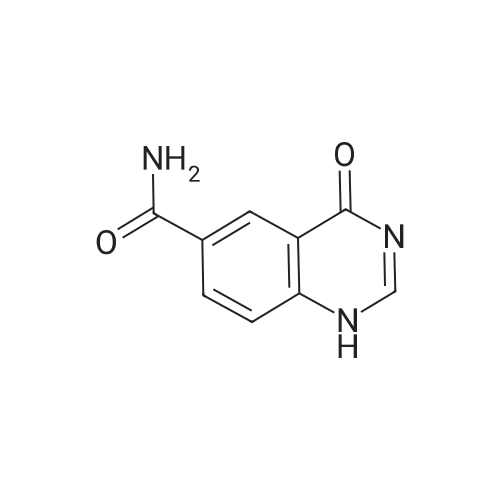
| 96.33% |
With thionyl chloride;N,N-dimethyl-formamide; at 78 - 80℃; for 7 - 8h;Heating / reflux; |
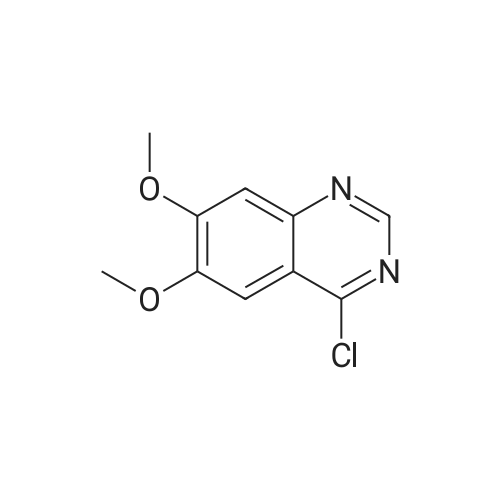
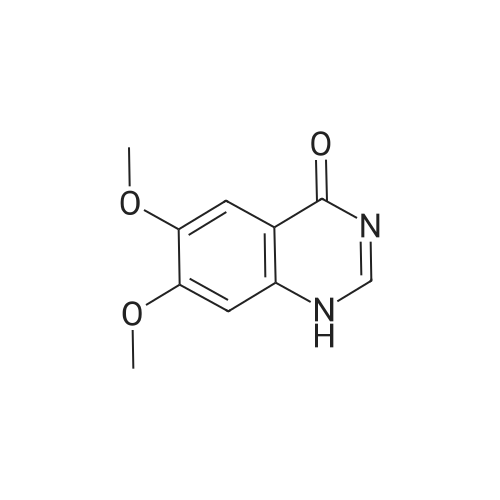
Example- 1:Ia) Preparation of 4-chloro-6, 7-dimethoxy-quinazolineDMF(Catalytic) compound-IVa720.0 g (6.05 mol) of thionyl chloride and 50.0g(0.243 mol) of 6,7-dimethoxy-3H- quinazoline-4-one were charged into a 2.0 L 4 necked round bottom flask connected to a mechanical stirrer, thermometer socket and double surface reflux condenser. Reaction mass temperature was raised to reflux temperature (78-800C). 20.0 ml of dimethyl formamide was added slowly at reflux temperature. Maintained the mass temperature at reflux for 7- 8 hours under stirring. Distilled off thionyl chloride completely under vacuum at below 7O0C. Cooled the mass temperature to 4O0C to 45C under nitrogen atmosphere 1000.0 ml of hexane was charged under stirring. Maintained the mass temperature at 40C to 45C for 30- 45 min. Cooled the mass temperature 25- 300C. Maintained the mass temperature at 25- 30C for 45-60 min under nitrogen atmosphere. Filtered the solid under nitrogen atmosphere. Solid was washed with 250.0 ml of hexane. Compound was dried in vacuum tray drier containing phosphorus pentoxide at 30-35C till the loss on drying is not more than 0.50% w/w. Obtained 52.5Og (yield is 96.33% by theory) of yellow coloured product. Melting range 214-220C. HPLC purity 96.5%.Spectral data : FT-IR (KBr) : 3060, 3041, 2951, 2838, 1618, 1562, 1505, 1429, 1360, 1336, 1232, 1163, 966, 878, 853, 806, 656, 615,493,471.ηNMR(DMSO-dδ):. δ Value(ppm):3.89-3.91(m)2(O-CH3)(6H), 7.37(s)Ar-Ha(lH), 7.46(s)Ar-Hb91H), 9.01(s) Hc (lH). <n="32"/>13CNMR: δ value (ppm): 56.55(2C)5 101.69(1C)5 105.95(1C)5 113.39(1C), 134.28(1C)5 148.01(1C)5 150.15(1C)5 155.68(1C)5 157.30(1C)5 157.80(1C) Mass : 225.6[M+1]5224.6[M] |
| 96.33% |
With thionyl chloride;N,N-dimethyl-formamide; at 78 - 80℃; |
Example-11a) Preparation of 4-chloro-6,7-dimethoxy-quinazoline 720.0 g (6.05 mol) of thionyl chloride and 50.0 g (0.243 mol) of 6,7-dimethoxy-3H-quinazoline-4-one were charged into a 2.0 L 4 necked round bottom flask connected to a mechanical stirrer, thermometer socket and double surface reflux condenser. Reaction mass temperature was raised to reflux temperature (78-80 C.). 20.0 ml of dimethyl formamide was added slowly at reflux temperature. Maintained the mass temperature at reflux for 7-8 hours under stirring. Distilled off thionyl chloride completely under vacuum at below 70 C. Cooled the mass temperature to 40 C. to 45 C. under nitrogen atmosphere 1000.0 ml of hexane was charged under stirring. Maintained the mass temperature at 40 C. to 45 C. for 30-45 min. Cooled the mass temperature 25-30 C. Maintained the mass temperature at 25-30 C. for 45-60 min under nitrogen atmosphere. Filtered the solid under nitrogen atmosphere. Solid was washed with 250.0 ml of hexane. Compound was dried in vacuum tray drier containing phosphorus pentoxide at 30-35 C. till the loss on drying is not more than 0.50% w/w. Obtained 52.50 g (yield is 96.33% by theory) of yellow coloured product.Melting range 214-220 C.HPLC purity 96.5%.Spectral data: FT-IR (KBr): 3060, 3041, 2951, 2838, 1618, 1562, 1505, 1429, 1360, 1336, 1232, 1163, 966, 878, 853, 806, 656, 615, 493, 471.1HNMR (DMSO-d6): δ Value (ppm): 3.89-3.91 (m) 2(O-CH3)(6H), 7.37 (s)Ar-Ha(1H), 7.46 (s)Ar-Hb91H), 9.01 (s) Hc (1H).13CNMR: 8 value (ppm): 56.55 (2C), 101.69 (1C), 105.95 (1C), 113.39 (1C), 134.28 (1C), 148.01 (1C), 150.15 (1C), 155.68 (1C), 157.30 (1C), 157.80 (1C)Mass: 225.6 [M+1], 224.6 [M] |
| 92% |
With thionyl chloride; N,N-dimethyl-formamide; for 7h;Reflux; |
A mixture of compound 2 (1.82 g, 8.8 mmol) and DMF (30 drops) in SOCl2 (50 mL) was refluxed for 7 h. The excess SOCl2 was removed by vacuum distillation. The residue was stirred with diethyl ether, filtered, washed, and dried to give compound 3 as a beige solid (1.83 g, 92%). A mixture of compound 3 and various amines in 2-propanol or DMF was refluxed. Upon completion of the reaction, it was cooled in an ice bath. The residue was filtered, washed, and dried to isolate the product. |
| 89.2% |
With trichlorophosphate; In dichloromethane; at 115℃; for 8h; |
To the dried 100mL round bottom flask, 6,7-dimethoxy-quinazolin-4-one (10g, 48.5mmol), POCl3 (25mL), were successively added refluxed under 115 C for 8h, TLC monitoring of the reaction (V ethyl acetate: V petroleum ether = 1: 4). After the reaction was complete. Under reduced pressure, most of the POCl3, was removed, cooled to 0C, dissolved in dichloromethane (DCM). The reaction mixture was poured into an appropriate amount of crushed ice and stirred, an appropriate amount of sodium bicarbonate solution was added and extracted three times twice before maintaining the solution was strongly acidic, the third time extraction, pH value was adjusted to 7, the organic layers combined, dried over anhydrous magnesium sulfate, and the DCM was removed under reduced pressure, to give a yellow flaky solid (9.69 g, yield 89.2% ). Product by thin layer chromatography (V petroleum ether / V ethyl acetate = 4: 1, R f = 0.45) in a purity of qualified. Without further purification, it was used directly in the next reaction. |
| 88% |
With thionyl chloride; N,N-dimethyl-formamide;Reflux; |
6,7-Dimethoxyquinazolin-4(3H)-one (20 g, 9.7 mmol) and 0.1 mL of /V,//-dimethylformamide were added to 50 mL of thionyl chloride. The resulting mixture was stirred at reflux for overnight. After cooled to room temperature, the solvent was removed in vacuo and saturated sodium carbonate solution was added to adjust the pH value to 8 at 0 C. The resulting mixture was extracted with dichloromethane and the combined organic layer was dried over anhydrous sodium sulfate. The solvent was removed in vacuo and the residue was purified by silica gel column chromatography (petroleum ether: ethyl acetate = 5: 1) to give 1.96 g (88%) of the tilte compound as a yellow solid. MS (ESIpos): m/z = 225 (M+H)+; LC-MS [Method 1] : Rt = 0.91 min. |
| 86.5% |
With trichlorophosphate; In toluene; for 3h;Reflux; |
0.1 mol6,7_ dimethoxy-quinazolin-4-one, 200mL toluene, 140mL phosphorus oxychloride were successively added single jar, magnetic stirrer, heated to reflux for 3h.After the reaction, the solvent and spin phosphorus oxychloride, adding an appropriate amount of crushed ice, ice with vigorous stirring to melt the whole, to give a yellow suspension, filtration, and the resulting solid was recrystallized from ethanol to give a white solid.Yield 86.5%. |
| 85% |
With thionyl chloride; In N,N-dimethyl-formamide;Reflux; |
The 4.12g (20mmol) 6,7-dimethoxy-quinazolinone by adding 500 ml single port in round low flask, then slowly added containing 1 drop of DMF in thionyl chloride of steams again 120 ml, reflux reaction, TLC detection after the reaction is finished, reducing pressure and evaporating excess of thionyl chloride, residue 300 ml ethyl acetate is dissolved, washing with saturated sodium bicarbonate solution to neutral, the organic layer dried anhydrous sodium sulfate, after concentrating column separation (V petroleum ether: V ethyl acetate: 4:1-2:1) to obtain 6,7-dimethoxy-4-chloro-quinazoline, yield: 85%, 1 HNMR (DMSO-d 6, 400MHz): 4.01 (s, 6H, 2CH 3), 7.38 (s, 1H), 7.45 (s, 1H), 8.88 (s, 1H); ESI-MS (100%): 224 ([M] +, 100). |
| 83.1% |
With thionyl chloride; In N,N-dimethyl-formamide; for 2h;Reflux; |
To the reactor was added 6,7-dimethoxy-4 (3H) -quinazolinone (5 g, 24.3 mmol)30 mL of thionyl chloride was added, 0.5 mL of N, N-dimethylformamide was added,The temperature was raised to reflux for 2 hours. After completion of the reaction,Rotate and evaporate out of the thionyl chloride, ice bath to the residue by adding saturated sodium bicarbonate solution,Stir until no gas is released. Add dichloromethane extraction, anhydrous sodium sulfate drying.The desiccant was removed by filtration,The solvent was removed by rotary evaporation to give 4.52 g of 4-chloro-6,7-dimethoxy-4 (3H) -quinazoline as a pale yellow solid,Yield 83.1%. |
| 82.2% |
With thionyl chloride; In N,N-dimethyl-formamide; for 1h;Reflux; |
A solution of compound 5 (1.7 g, 8.2 mmol) in SOCl2 (15 mL) containing 0.05 mL ofN,N-dimethylformamide (DMF) were heated to reflux in 100 mL flask for 1 h. Excess SOCl2 wasdistilled in vacuum and the resulting residue was adjusted to pH value 8-10 with aqueous Na2CO3.1.53 g of product was got after filtered as yellow solid, yield: 82.2%, m.p. 180.4-182.0 C. |
| 82% |
With trichlorophosphate; at 120℃; for 6h;Inert atmosphere; |
A mixture of compound 6 (4.00 g, 19.40 mmol) and phosphorylchloride (40 mL) was stirred at 120 C for 6 h. Thesolvent was removed in vacuo and the residue was dissolvedin ice-H2O and washed with saturated aqueousNaHCO3 solution (three times) followed by brine. Theorganic layer was dried over MgSO4, filtered, and the solventwas evaporated in vacuo. Recrystallizaton from EtOHafforded the chlorinated product 7 (3.58 g, 82%) as a yellowsolid. Analytical data for compound 7 are in agreement withthe literature (VanBrocklin et al. 2005). |
| 81.7% |
With thionyl chloride; In N,N-dimethyl-formamide; for 4h;Reflux; |
A stirred mixture of 6 (2.0 g, 0.010 mol), thionyl chloride (30 mL) and N,N-dimethylformamide (0.6 mL) was heated under reflux for 4 h. The solvent was removed in vacuo to obtain the off-white crude product and the crude product was recrystallized in DMF to obtain the compound 7 (1.81g, yield 81.7%), m.p.: 178 C. IR (cm-1): ν 3431, 1618, 1560,1508, 1412, 1348, 1234, 1161, 968, 850, 698; 1H-NMR (DMSO-d6) δ (ppm): 8.88(1H, s, 2-H),7.46(1H, s, 5-H), 7.41 (1H, s, 8-H), 4.00 (6H, s, -OCH3); 13C-NMR (DMSO-d6) δ (ppm): 158.82 (2-C), 155.05 (7-C), 149.34 (6-C), 145.86 (9-C), 138.85 (10-C), 114.73 (5-C), 105.52 (8-C), 104.11 (C-4),56.16 (-OCH3), 55.94 (-OCH3). |
| 80% |
With trichlorophosphate; at 105℃; for 2h; |
Example 4 4-chloro-6,7-dimethoxy-quinazoline In a 100mL flask equipped with a reflux condenser, 6,7-dimethoxy-quinazolin-4-one 4.5g(20mmol), phosphoryl chloride (45ml) were added. The mixture was stirred at 105 for 2h, and then was poured into 100mL of ice water carefully, and off-white squama solid was deposited slowly, which was filtered, dried and identified as the title compound. Yield: 80%. 1H-NMR (400MHz, DMSO): δ8.89(1H, s), 7.47(1H, s), 7.41(1H, s), 4.02(3H, s), 4.00(3H, s). |
| 80% |
With trichlorophosphate; at 105℃; for 2h; |
Example 4 4-chloro-6,7-dimethoxy-quinazoline In a 100 mL flask equipped with a reflux condenser, 6,7-dimethoxy-quinazolin-4-one 4.5 g (20 mmol), phosphoryl chloride (45 ml) were added. The mixture was stirred at 105 C. for 2 h, and then was poured into 100 mL of ice water carefully, and off-white squama solid was deposited slowly, which was filtered, dried and identified as the title compound. Yield: 80%. 1H-NMR (400 MHz, DMSO): δ8.89(1H, s), 7.47 (1H, s), 7.41 (1H, s), 4.02 (3H, s), 4.00 (3H, s). |
| 75.8% |
With triethylamine; trichlorophosphate; In toluene; for 4h;Inert atmosphere; Reflux; |
In a 100 mL three-necked flask, 1.1 g (4.6 mmol) of 6,7-trimethoxyquinazolinone, 2 mL of phosphorus oxychloride (21.8 mmol) and 12 mL of toluene were mixed and then 4 mL of tris Ethylamine, take white smoke, argon reflux began under the protection of the reaction, stop after 4 h, with dilute ammonia water to adjust the pH value to about 7, with dichloromethane (20 mLX3) extraction, after desolventizing yellow-green solid column layer Analysis (PE: ΕΑ = 5: 1, V / V) purified to give a white solid 0.9 g, yield: 75.8%, melting point: 115_1170C. |
| 71% |
With trichlorophosphate; at 0℃; for 9h;Reflux; |
General procedure: To phosphoryl chloride (30 ml, 0.32 mol), the selected quinazolin-4(3H)-one (10 mmol) was added at 0 C and stirred for 10 min.The resulting mixture was then refluxed for 9 h. After removal ofexcess solvent, the residue was dissolved in ice-water (50 ml)and the solution was neutralized with ammonium hydroxide.The solution was extracted three times with dichloromethane(50 ml). The organic layer was washed with brine (100 ml), driedover MgSO4, and the solvent was removed under reduced pressure.The formed solid was recrystallized from ethanol. For further usethe structure of the compounds were confirmed by NMR. |
| 70% |
With thionyl chloride; In N,N-dimethyl-formamide; for 3h;Reflux; |
General procedure: A mixture of 4-hydroxyquinazoline (50 mmol, 1.0eq) and thionyl chloride(200mL) containing DMF (0.4mL) was refluxed for 3 h. The reaction was cooled, excess thionyl chloride was removed under reduced pressure and the residue was diluted in dichloromethane (500 mL). The solution was sequentially washed with saturated aqueous sodium hydrogen carbonate solution (2 x 250 mL) and brine, respectively, dried over anhydrous Na2SO4 and then concentrated organic phase under reduced pressure to provide the compound as a white solid (4a) [44]. The procedure described for the synthesis of compound 4a can also be applied to the synthesis of compounds 4b-4c. |
| 70% |
With thionyl chloride; In N,N-dimethyl-formamide; for 6h;Reflux; |
With a thermometer,6,7-Dimethoxyquinazolin-4(3H)-one (10.3 g, 50 mmol) was dissolved in thionyl chloride (200 mL) in a three-necked flask of a magnetic stirring and reflux condenser.DMF (0.4 ml) was added dropwise, and the mixture was heated under reflux for 6 hours.After cooling, excess thionyl chloride was distilled off under reduced pressure, and the residue was azeotroped with toluene (50 mL) to remove the thionyl chloride, and then dichloromethane (500 mL).It was washed twice with a saturated aqueous solution of sodium hydrogencarbonate and water, and the organic phase was dried over magnesium sulfate.The solvent was evaporated in vacuo to give a white solid IV-2,(7.84g, 70%), |
| 64% |
With trichlorophosphate; for 9h;Reflux; |
General procedure: The selected quinazolin-4(3H)-one (10 mmol) was mixed with 10 ml phosphoryl chloride and was then stirred under reflux for 9 h. After completion of the reaction the solvent was evaporated under reduced pressure. Ice-water was added to the residue and the formed precipitatewas neutralized with ammonium hydroxide and was filtered off. 5.3.2 4-Chloro-6,7-dimethoxyquinazoline (6) The product was synthesized from compound (2) and recrystallized from ethanol to yield pale yellow solid (64%). 1H NMR (500 MHz, DMSO-d6) δ 8.86 (s, 1H), 7.42 (s, 1H), 7.36 (s, 1H), 4.00 (s, 3H), 3.98 (s, 3H). 13C NMR (126 MHz, DMSO-d6) δ 158.00, 156.88, 152.31, 151.53, 148.72, 118.72, 107.00, 102.37, 56.65, 56.30. |
| 27% |
With thionyl chloride; In N,N-dimethyl-formamide; |
A mixture of 6,7-dimethoxy-3,4-dihydroquinazolin-4-one (2.06 g), thionyl chloride (20 ml) and DMF (1 drop) was stirred and heated to reflux for 2 hours. The mixture was evaporated and the residue was partitioned between ethyl acetate and a saturated aqueous sodium hydrogen carbonate solution. The organic phase was washed with water, dried over magnesium sulphate, filtered and evaporated to dryness. The residue was purified by column chromatography using increasingly polar mixtures of methylene chloride and ethyl acetate as the eluant to give 4-chloro-6,7-dimethoxyquinazoline (0.6 g, 27%). |
| 27% |
With thionyl chloride;N,N-dimethyl-formamide; for 2h;Heating / reflux; |
To a portion (2.06g) of the material so obtained were added thionyl chloride (20ml) and DMF (1 drop) and the mixture stirred and heated at reflux for 2 hours. Excess thionyl chloride was removed by evaporation and the residue was partitioned between ethyl acetate and a saturated aqueous sodium hydrogen carbonate solution. The organic phase was washed with water, dried (MgSO4) and the solvent removed by evaporation. The residue was purified by column chromatography using increasingly polar mixtures of methylene chloride and ethyl acetate as eluent to give 4-chloro-6,7-dimethoxyquinazoline (0.6g, 27%). |
|
With thionyl chloride; In N,N-dimethyl-formamide; for 2h;Heating / reflux; |
Example 6 6,7-Dimethoxy-3,4-dihydroquinazolin-4-one (290mg, 1.4mmol) was suspended in thionyl chloride (5ml) and DMF (2 drops) and heated at reflux for 2 hours. The thionyl chloride was evaporated under vacuum and the residue azeotroped with toluene three times to give 4-chloro-6,7-dimethoxyquinazoline. |
|
|
Compound 4 was refluxed with phosphorus oxytrichloride to give 4-chloro-6,7-dimethoxyquinazoline (compound 5) in good yield. |
|
In dichloromethane; sodium carbonate; trichlorophosphate; |
4-Chloro-6,7-dimethoxyquinazoline 5. A suspension of 6,7-dimethoxyquinazoline-4(3H)-one 4 (12.36 g, 60 mmol) in POCl3 (250 mL) was heated under reflux for 4 hr, when a clear solution was obtained. The POCl3 was removed under reduced pressure, and the residue was dissolved in a mixture of CH2Cl2 and aqueous Na2CO3. The organic layer was dried and the solvent removed to give 4-chloro-6,7-dimethoxyquinazoline 5 (11.2 g, 83%); m.p. 259.0-263.0 C.; 1H NMR (DMSO-d6): d 8.75(s, 1H, 2-H), 7.53(s, 1H, 5-H), 7.25(s, 1H, 8H), 3.91(s, 3H, -OCH3), 3.89(s, 3H, -OCH3); IR (KBr) umax: 2963, 2834, 1880, 1612, 1555, 1503, 1339, 1153, 962 cm-1. GC/MS m/z 224(M+, 100), 209(M+ -CH3, 9.4), 189(19.39), (69(10.55). |
|
|
Compound 4 was refluxed with phosphorus oxytrichloride to give 4-chloro-6,7-dimethoxyquinazoline (compound 5) in good yield. |
|
With thionyl chloride;N,N-dimethyl-formamide; |
Preparation of the 4-(3-ethynyl-phenyl)amino- 6,7-dimethoxy-quinazoIine 3 was achieved in four steps from the commercially available ester 1. (Knesl et al. (2006) Molecules, 11:286-297; Wright, et al. (2001) Bio-org. Med. Ch em. Lett., 1 /; 17-21. |
|
With trichlorophosphate; In acetonitrile; at 110℃; for 0.0833333h;Microwave irradiation; |
General procedure: A solution of 6,7-dimethoxyisoquinolin-1(2H)-one(200 mg, 0.97 mmol), phosphoryl trichloride (0.268 mL, 2.92 mmol) inacetonitrile (5 mL) was stirred at 110 C for 5 minutes under microwaveirradiation. The reaction was quenched with a saturated aqueous sodium bicarbonatesolution and stirred at ambient temperature for 1 h. It was filtered throughcelite and washed with ethyl acetate. The filtrate was concentrated to dryness.The crude material was purified by flash chromatography, eluting with heptanesand ethyl acetate (1:0 to 0:1) to give the desired product as a gum (99 mg,45.4 %). |
|
With thionyl chloride; N,N-dimethyl-formamide; for 2h;Reflux; |
To a stirred solution of compound 1 (10 mmol) in thionyl chloride (3 mL), DMF was added (2-3 drops) slowly. The reaction mixture was heated to reflux and stirred for about 2 h. Excess thionyl chloride was distilled off and the reaction mixture was quenched in ice with efficient stirring. The precipitate was filtered, washed with ice-water and the crude material was dissolved in chloroform and filtered to remove insoluble impurities. Organic layer was concentrated under vacuum to obtain compound 2. |
|
With trichlorophosphate; |
(9.7 mmol) of phosphorus oxychloride was added. After 4-9 h of reaction, the remaining phosphorus oxychloride was removed by steaming and extracted with water and methylene chloride to give crude product. The silica gel column was used to give compound 3. |
|
With thionyl chloride; N,N-dimethyl-formamide; for 8h;Reflux; |
General procedure: A mixture of 4-quinazolone analogues 2a-2j (8.0 mmol) in SOCI2 (27.4 mL) containing DMF (2 drops) was refluxed for 8 h. SOCI2 was removed under reduced pressure and the residue was dissolved in DCM. The solution was washed with saturated NaHCO3 solution and brine, respectively, dried over anhydrous Na2S04 and then concentrated under reduced pressure to yield the compounds 3a-3j (65.1-88.9percent yield) as white or off-white solid. |
| 13 g |
With thionyl chloride; for 3h;Inert atmosphere; Reflux; |
In a 250 mL three-necked flask, 20 g of 6,7-dimethoxyquinazolin-4-one was added, followed by addition of 100 mL of thionyl chloride,Nitrogen protection reaction system, heated to reflux reaction, reaction 3h,The thionyl chloride was distilled off to give 4-chloro-6,7-dimethoxyquinazoline13g. |
| 13 g |
With thionyl chloride; for 3h;Inert atmosphere; Reflux; |
A 250 mL three-necked flask was charged with 20 g of 6,7-dimethoxyquinazolin-4-one and 100 mL of thionyl chloride, and nitrogenGas protection reaction system, heated to reflux reaction, the reaction 3h, evaporated to give 4-chloro-6,7-dimethoxyquinazole13 g |
|
With thionyl chloride; In N,N-dimethyl-formamide; for 5h;Reflux; |
General procedure: A mixture of 4-hydroxyquinazoline (0.02 mol) in SOCl2 (20 mL)containing DMF (2 drops) was refluxed for 5 h. SOCl2 was removedunder reduced pressure, and the residue was dissolved in dichloromethane(DCM). The solution was washed with NaHCO3 solutionand brine, dried over anhydrous Na2SO4, and concentrated under reducedpressure to obtain the desired compound as a yellow solid. |
| 85.1%Chromat. |
With thionyl chloride; In N,N-dimethyl-formamide; at 79 - 81℃; for 6h; |
add dry 6,7-dimethoxy-3H-quinazolin-4-one (compound 1, 82.43g, 0.4mol), thionyl chloride (300mL), dry DMF (15mL), install a reflux condenser, install a drying tube above the condenser, heat, control the temperature at 79C -81C, stir for 6h, After cooling slightly, the thionyl chloride was removed under reduced pressure, the residue was transferred to a beaker, methanol (100 mL) was slowly added, and after stirring for 10 min, the solvent was removed under reduced pressure to remove the remaining trace of thionyl chloride to obtain a yellow-red solid , Add ether (400mL) to the above solid, stir for 0.5h, filter with suction, wash the filter residue with ether twice, Obtained a beige solid, the product, which was tested by thin layer chromatography TLC, with a purity of 98.5% and a yield of 85.1%. |
|
With N,N-dimethyl-aniline; trichlorophosphate; at 20℃; for 5h;Reflux; |
To a suspension of the starting material quinazolinone 1a(146 mg, 1 mmol, 1eq) in phosphorus oxychloride (280 μL, 3 mmol,3eq) was added N,N-dimethyl aniline (135 μL, 128 mmol, 1.05 eq)dropwise at ambient temperature. Then the reaction mixture washeated to reflux for 5 h. The excess phosphorus oxychloride wascollected under reduced pressure. To the slurry was added crushedice and stirred for 10 min. The precipitate was collected by filtration,and then dried under vacuum to afford 4-choloroquinazoline(2a) as off-white solid (122 mg, 74%). The product was used for thenext step without further purification. This procedure was alsoapplied to the preparation of intermediates 2b-g. |
|
With trichlorophosphate; at 100℃; for 2h; |
General procedure: To a solution of intermediates 7a-7i (1 equiv.) and ammonium formate (3 equiv.) in EtOH was added trimethyl orthoformate (3 equiv.). The mixture was heated under reflux for 5-8h. After the addition was completed, TLC analysis indicated the reaction was complete. After cooled to room temperature, the mixture was filtered, and the solid was collected and dried to give a crude intermediates 8a-8i for the next step. A solution of the corresponding intermediates 8a-8i (1 equiv.) in POCl3 (10 equiv.) was stirred at 100C for 2h. After the addition was completed, TLC analysis indicated the reaction was complete. The mixture was cooled to room temperature, and the solvent was removed under reduced pressure to give a crude intermediates 9a-9i for the next step. Then, a solution of corresponding intermediates 9a-9i (1 equiv.), methyl 4-(aminomethyl)-benzoate hydrochloride (1 equiv.) and DIPEA (4 equiv.) in IAP was stirred at 90C for 6-8h, at which time TLC analysis indicated the reaction was completed. After cooled to room temperature, the mixture was filtered, and the solid was collected to give intermediates 10a-10i, which was used directly in the next step without purification. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping