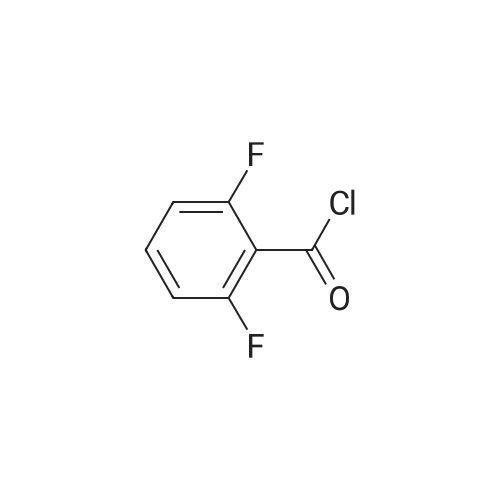
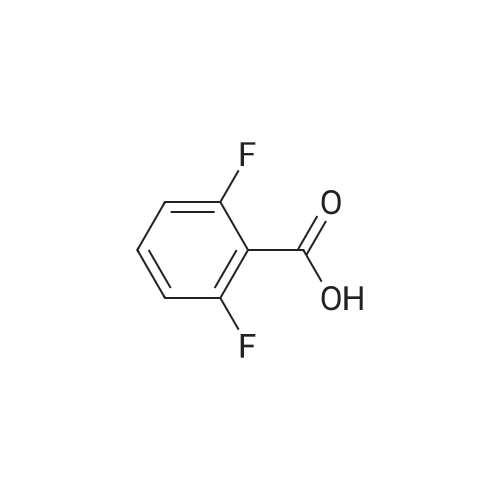
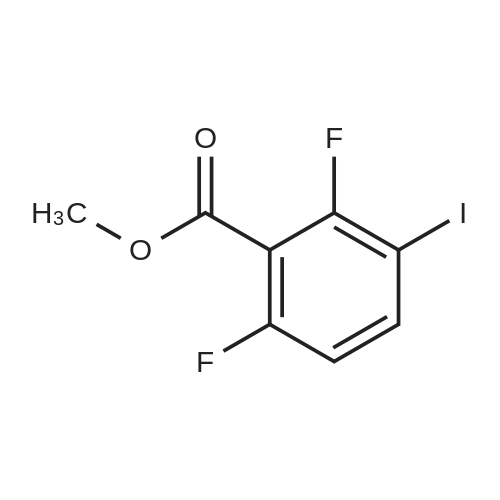
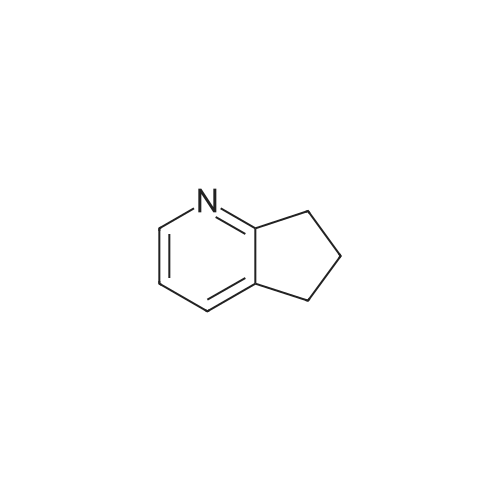
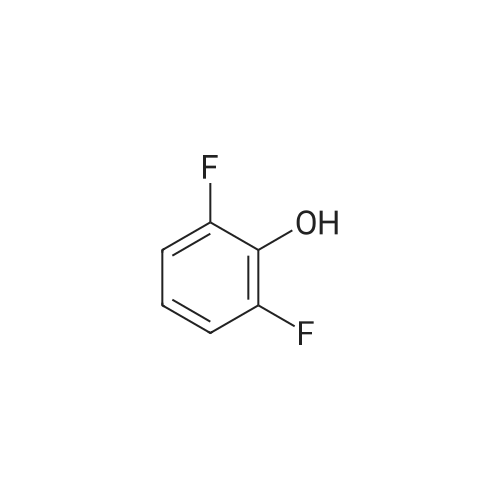
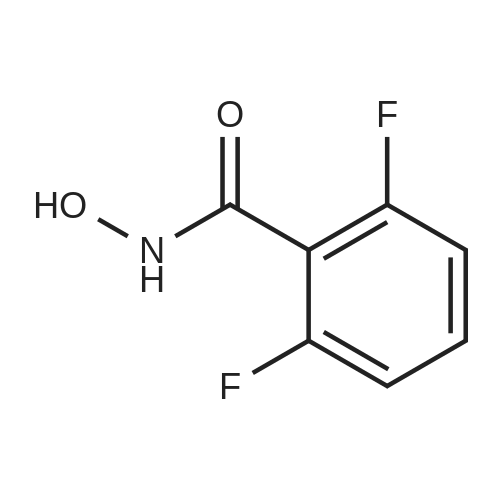
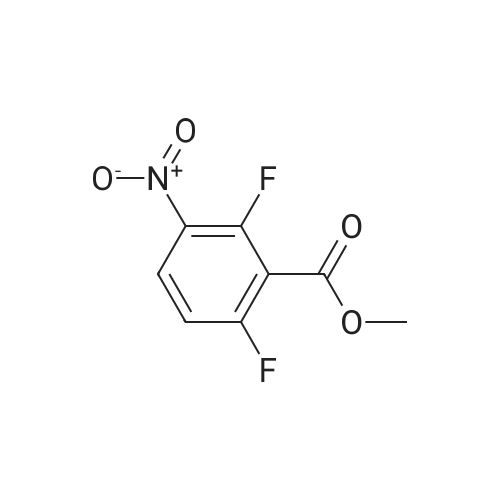
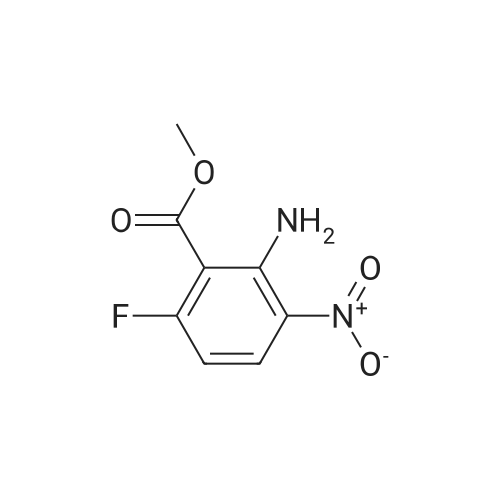
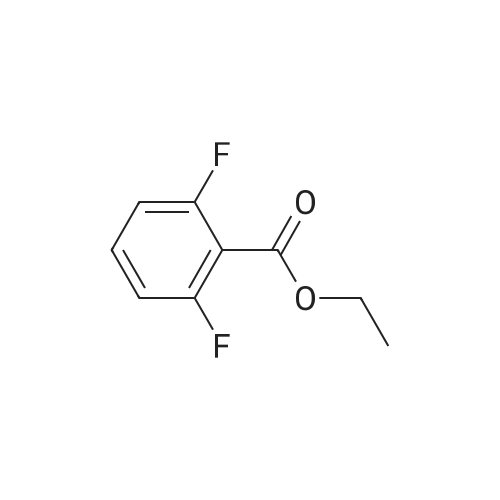
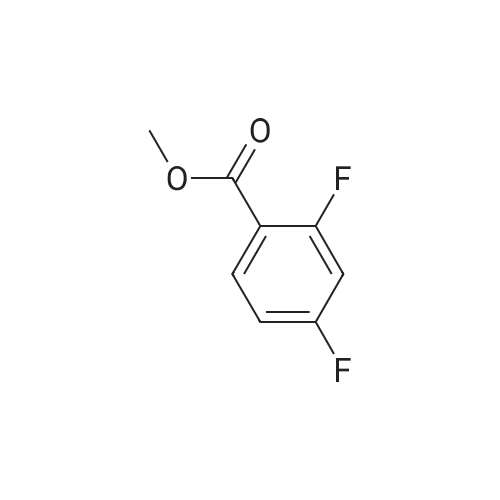
| 80.6% |
With sulfuric acid; nitric acid; at 0℃; for 0.5h; |
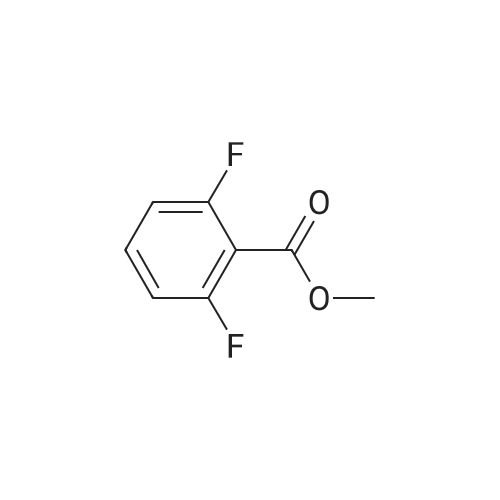
Fuming nitric acid (1 1 g, 174 mmol) was added to a solution of methyl 2,6- difluorobenzoate (25 g, 145 mmol) in concentrated sulfuric acid (50 ml_) at 0 C, and the reaction was stirred for 30 min at 0C. The reaction mixture was poured over ice-water. The precipitate was filtered to give the title compound 25.1 g (80.6 % yield) 1H NMR (400 MHz, CDCI3) delta ppm 8.13-8.20 (m, 1 H), 7.02-7.10 (m, 1 H), 3.93 (s, 3H). |
| 64% |
With sulfuric acid; nitric acid; at 20℃; for 1h;Cooling with ice; |
Sulfuric acid (37 mL) was slowly added to nitric acid (20 mL) under ice-cooling, and added <strong>[13671-00-6]methyl 2,6-difluorobenzoate</strong> (25.7 g,149 mmol), The reaction was gradually warmed to room temperature and stirring was continued for 1 hour. The reaction system was poured into ice-water and filtered to obtain a white solid compound P'(20.7 g, yield 64%). |
| 64% |
With sulfuric acid; nitric acid; at 20℃; for 1h;Cooling with ice; |
Sulfuric acid (37 mL) was slowly added to nitric acid (20 mL) under ice-cooling, and <strong>[13671-00-6]methyl 2,6-difluorobenzoate</strong> (25.7 g, 149 mmol) and the reaction was gradually allowed to warm to room temperature. Stirring was continued for 1 hour, The reaction system was poured into ice-water and filtered to give a white solid compound d (20.7g, yield 64%) |
|
With sulfuric acid; nitric acid; In water; at 0 - 20℃; for 0.583333h; |
To fuming nitric acid (3.87 mL, 86.6 mmol) at O 0C was slowly added concentrated sulfuric acid (7.27 mL, 136.4 mmol). After stirring for 5 min., <strong>[13671-00-6]methyl 2,6-difluorobenzoate</strong> (3.90 mL, 29.0 mmol) was added and the reaction mixture was allowed to warm to ambient temperature. After 30 min., the reaction mixture was poured into ice- water, and extracted thrice with dichloromethane. The combined organic portions were washed with saturated aqueous sodium bicarbonate, dried over MgSO4, filtered, and concentrated in vacuo to afford a colorless oil. MS(ES+) m/e 218 [M+eta]+. Upon standing, the oil solidified to a white solid, which was dissolved in ethanol (50.0 mL) and treated with ammonium hydroxide (1.0 mL, 29 % aqueous solution) at ambient temperature. After 4 h, additional ammonium hydroxide (0.8 mL, 29 % aqueous solution) was added and the reaction mixture was stirred overnight. The solution was concentrated and the residual solid was washed with isopropanol, filtered, washed with water, and dried in vacuo to afford the title compound (5.69 g, 92%) as a yellow solid. 1H NMR (400 MHz, DMSO-iie) delta ppm 8.33 (dd, J=9.5, 5.7 Hz, 1 H), 8.11 (br. s., 2 H), 6.62 (t, J=9.9 Hz, 1 H), 3.89 (s, 3 H). MS(ES+) m/e 215 [M+H]+. |
|
With sulfuric acid; nitric acid; at 0 - 20℃; |
1a) Methyl 2-amino-6-fluoro-3-nitrobenzoate To fuming nitric acid (3.87 mL, 86.6 mmol) at 0 C. was slowly added concentrated sulfuric acid (7.27 mL, 136.4 mmol). After stirring for 5 min., <strong>[13671-00-6]methyl 2,6-difluorobenzoate</strong> (3.90 mL, 29.0 mmol) was added and the reaction mixture was allowed to warm to ambient temperature. After 30 min, the reaction mixture was poured into ice-water and extracted thrice with dichloromethane. The combined organic portions were washed with saturated aqueous sodium bicarbonate, dried over MgSO4, filtered, and concentrated in vacuo to afford a colorless oil. MS (ES+) m/e 218 [M+H]+. Upon standing, the oil solidified to a white solid, which was dissolved in ethanol (50.0 mL) and treated with ammonium hydroxide (1.0 mL, 29% aqueous solution) at ambient temperature. After 4 h, additional ammonium hydroxide (0.8 mL, 29% aqueous solution) was added and the reaction mixture was stirred overnight. The solution was concentrated and the residual solid was washed with isopropanol, filtered, washed with water, and dried in vacuo to afford the title compound (5.69 g, 92%) as a yellow solid. 1H NMR (400 MHz, DMSO-d6) delta ppm 8.33 (dd, J=9.5, 5.7 Hz, 1H) 8.11 (br. s., 2H) 6.62 (t, J=9.9 Hz, 1H) 3.89 (s, 3H). MS (ES+) m/e 215 [M+H]+. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping