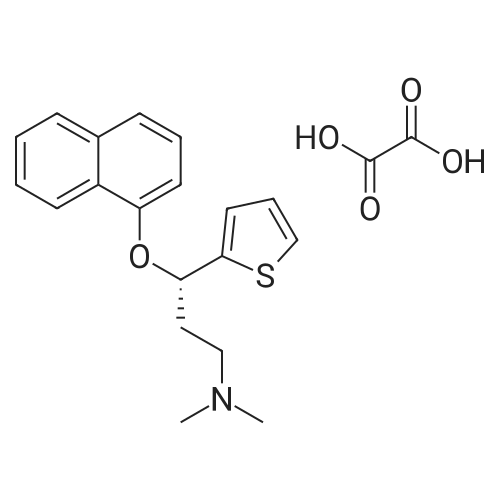
| 92.1% |
With glucose dehydrogenase; D-glucose; Rhodosporidium toruloides carbonyl reductase 9 from Escherichia coli; NADPH; sodium hydroxide; In aq. phosphate buffer; at 30℃; for 4h;pH 7;Enzymatic reaction;Kinetics; |
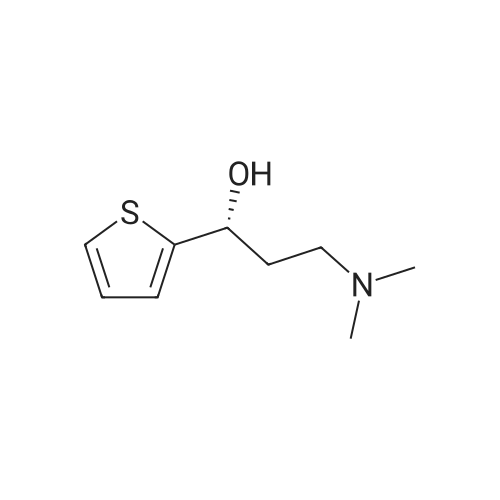
In a 2-L bioreactor, 2a (1000mM), glucose (6000mM), NADP+ (0.75mM), and sonicated cells of co-expressing E. coli/RtSCR9-GDH (10gDCWL-1) were mixed in a total volume of 1L phosphate buffer (100mM, pH 7.0). The resulting mixture was stirred at 30C with 200rpm shaking for 240min. The pH was maintained at 7.0 with 2M NaOH during the reaction. After reaching completion, the pH was adjusted to 11-12 followed by extraction using ethyl acetate. The combined organic phases were dried over anhydrous sodium sulfate and concentrated to give (S)- 3a. Yield: 92.1%. 1H NMR (500MHz, DMSO-d6): delta=7.36 (dd, J=4.9, 1.2Hz, 1H), 6.93 (m, 2H), 5.79 (s, 1H), 4.86 (m, 1H), 2.30 (qt, J=12.0, 7.1Hz, 2H), 2.12 (s, 6H), 1.79 (m, 2H). 13C NMR (126MHz, DMSO-d6): delta=150.72, 126.40, 123.79, 122.51, 67.39, 55.96, 45.15, 36.92. [alpha]25D=-4.9 (c=10mg/mL, ethyl acetate). |
|
With hydrogen; sodium hydrogencarbonate; (2R,4R)-4-(dicyclohexylphosphino)-2-(diphenylphosphino-methyl)-N-methyl-aminocarbonyl-pyrrolidine;di-mu-chloro-bis(1,5-cyclooctadiene)dirhodium; In methanol; water; at 30℃; under 75007.5 Torr; for 20h; |
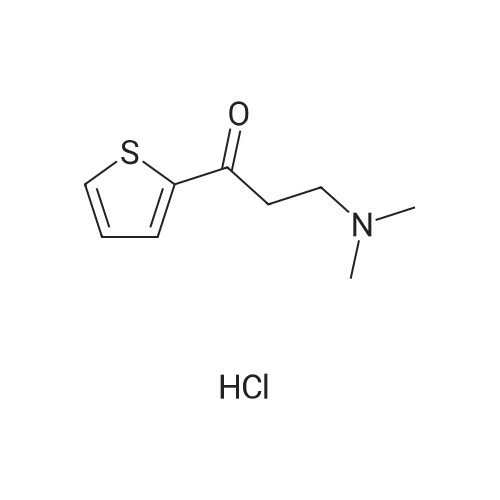
70 g (0.32 mol) of 1-(N,N-dimethylamino)-3-(2-thienyl)-propan-3-one-hydrochloride are suspended in 630 ml of methanol and 70 ml of water under nitrogen; 16.5 mg bis-(1.5-cyclooctadiene)-dirhodium(I)-dichloride, 34.9 mg (2R, 4R)-4-dicyclohexylphosphino)-2-(diphenylphosphino-methyl)-N-methyl-aminocarbonyl) pyrrolidine and 140 mg sodium hydrogen carbonate are added and the suspension is hydrogenated at 30 C. and 100 bar hydrogen pressure for about 20 hours. Then the reaction mixture is evaporated down and the residue obtained is divided between 350 ml of water and 250 ml organic solvent (toluene or dichloromethane). The pH is adjusted to 1.6 with 32% hydrochloric acid and the mixture is stirred for 10 minutes, then the aqueous phase is separated off. The organic phase is again combined with 250 ml of water, stirred and the aqueous phase is again separated off. The combined aqueous phases are adjusted to pH 9.0 with 400 ml organic solvent and 45% sodium hydroxide solution, stirred, and then the phases are separated. The aqueous phase is extracted again with 200 ml solvent and the combined organic phases are evaporated down at 60 C. and 5 mbar. The yield of crude product is 50.0 g (85% of theory), chemical purity >98% (NMR). The crude product is recrystallised from 150 ml n-heptane, washed with another 50 ml of n-heptane and dried overnight at 40 C. and 5 mbar. 46.8 g (79% of theory) of S-N, N-dimethyl-3-hydroxy-3-(2-thienyl)-propylamine are obtained as a white solid, purity >98% (NMR), enantiomeric purity 94% (HPLC), melting point 76-78 C. |
|
|
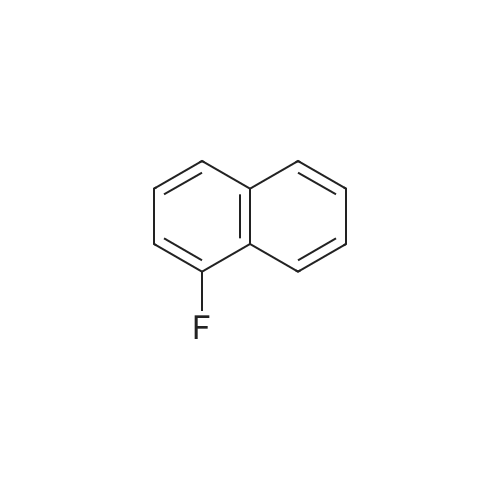
The duloxetine alkyl carbamate represented by the formula (Ib) was synthesized by a known method (steps 1 to 4 of the above scheme).Specifically, 2-acetylthiophene (63.1 g; 0.5 mol), dimethylamine hydrochloride (53.0 g; 0.65 mol), paraformaldehyde (19.8 g; 0.22 mol) and ethanol (80 ml) (1 ml) to give 3-dimethylamino-1- (2-thienyl) -1-propanone hydrochloride,Reduction with sodium borohydride, resolution with optically active mandelic acid yielded optically active (S) N, N-dimethyl-3- (2-thienyl) -3-hydroxypropylamine.Next, by reaction of 1-fluoronaphthalene with sodium hydride, N, N-dimethyl-duloxetine was obtained.Subsequently, the reaction with the corresponding alkyl chloroformate (4.6 ml; 48.5 mmol) gave the duloxetine alkyl carbamate represented by the above formula (Ib). The ethanol solution (10 mL) of the potassium hydrate (87% of purity, 2.16g;33.5mmol) was added to the toluene solution (10 mL) of the obtained duloxetine ethyl carbamate (Ib) and (2.5g;6.7mmol) at the room temperature. It heated until it flowed back at 85 to 90 degree C about mixed liquor, and the solvent was distilled off until the internal temperature became 100 degrees C from there. Repeating distilling off of a solvent furthermore, in the range of 95 to 100 degree C, temperature was maintained and it stirred for 2 hours. It cooled to the room temperature and washed the organic layer obtained by adding water (10 mL) and ethyl acetate (10 mL) with water (20 mL). With sodium sulfate, it dried, concentration drying was filtered and carried out, and the compound of the title was obtained at 1.96 g (yield: 98.4%, optical isomer:0.16%).[0054]1H-NMR(400-MHz, DMSO-d6):8.23-8.20 (m, 1H), 7.86-7.82 (m, 1H), 7.53-7.49 (m, 2H), 7.44-7.41 (m, 2H), 7.33 (t, 1H), 7.21 (dd, 1H), 7.05(d,1H),6.97(dd,1H),5.99(t,1H),2.62(2H,t)2.26(3H,s),2.36-2.29(m,1H),2.12-2.03(m,1H) |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping