| 94% |
|
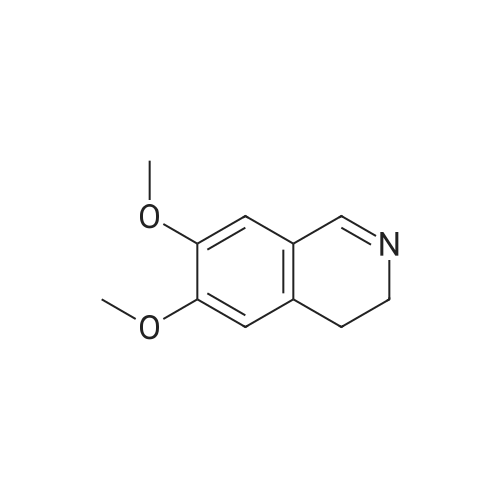
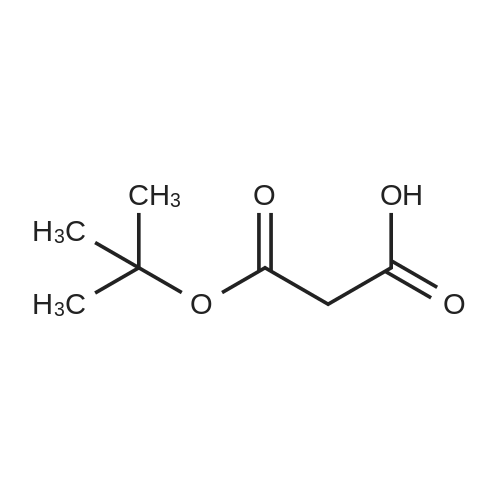
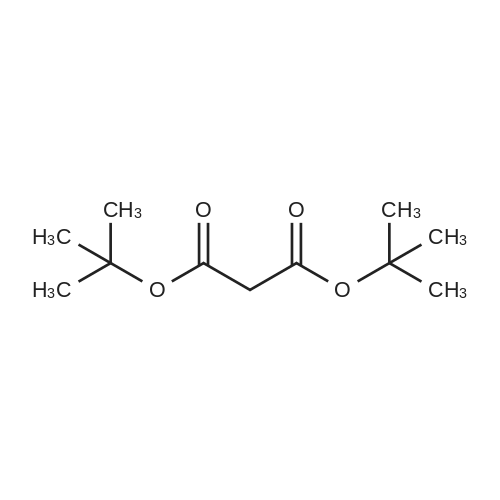
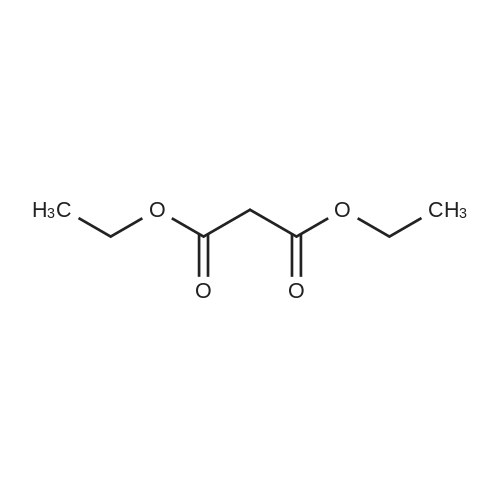
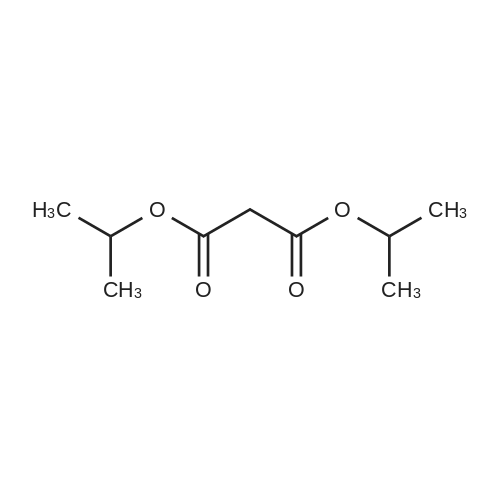
Example 1 Preparation of Protected Diester 2 The dihydroisoquinoline 1 (1.0 eq.) and Boc anhydride (1.5 eq.) were dissolved in CH2Cl2 at room temperature to provide a 1.5 M solution with respect to the dihydroisoquinoline. The mixture was allowed to stir for 30 min. Following the allotted time, the reaction mixture was cooled to 0 C. and then diisopropylmalonate (1.5 eq.) followed by a pre-chilled solution of the Pd catalyst (0.008 eq.) in dichloromethane were added successively to the reaction mixture to provide a final reaction concentration of 0.84 M with respect to the starting dihydroisoquinoline. The reaction mixture was allowed to continue stirring at 2.5 C. for 15 h. Following this time EtOAc and brine were added to the reaction mixture. The aqueous layer was extracted with three portions of EtOAc and the combined organic layers were dried (Na2SO4), filtered, and concentrated under reduced pressure to provide the crude product. The crude material was dissolved in a minimal amount of dichloromethane and purified by flash chromatography on SiO2 (15-30% EtOAc-hexanes, elution was observed at 285 nm and 228 nm). The product 2 was a colorless solid that existed as a mixture of rotamers in solution at room temperature 94%: [α]26D-69.0 (c 0.21, CHCl3); 1H NMR (CDCl3) δ 0.81-1.02 (m, 6H), 1.06-1.17 (m, 6H), 1.23-1.38 (m, 9H), 2.51-2.63 (m, 1H), 2.64-2.77 (m, 1H), 3.20-3.29 (m, 0.6H), 3.32-3.41 (m, 0.4H), 3.51-3.58 (m, 1H), 3.62-3.70 (m, 6H), 3.70-3.76 (m, 0.4H), 3.91-4.01 (m, 0.6H), 4.65-4.82 (m, 1H), 4.83-4.98 (m, 1H), 5.71 (apparent d, J=5.7 Hz, 0.6H), 5.78 (apparent d, J=7.9 Hz, 0.4H), 6.42-6.49 (m, 1H), 6.77 (s, 0.6H), 6.81 (s, 0.4H); 13C NMR (CDCl3) δ 21.02, 21.09, 21.18, 21.32, 27.24, 27.95, 28.02, 37.60, 39.34, 52.11, 52.83, 55.48, 55.52, 59.28, 60.08, 68.58, 68.76, 68.82, 79.46, 80.03, 110.09, 110.73, 111.13, 126.11, 126.18, 126.37, 127.07, 146.81, 146.87, 147.93, 153.86, 154.30, 166.29, 166.78, 166.94, 167.06. |
| 94% |
|
Example 1Preparation of Protected Diester 2 The dihydroisoquinoline 1 (1.0 eq.) and Boc anhydride (1.5 eq.) were dissolved in CH2Cl2 at room temperature to provide a 1.5 M solution with respect to the dihydroisoquinoline. The mixture was allowed to stir for 30 min. Following the allotted time, the reaction mixture was cooled to 0 C. and then diisopropylmalonate (1.5 eq.) followed by a pre-chilled solution of the Pd catalyst (0.008 eq.) in dichloromethane were added successively to the reaction mixture to provide a final reaction concentration of 0.84 M with respect to the starting dihydroisoquinoline. The reaction mixture was allowed to continue stirring at 2.5 C. for 15 h. Following this time EtOAc and brine were added to the reaction mixture. The aqueous layer was extracted with three portions of EtOAc and the combined organic layers were dried (Na2SO4), filtered, and concentrated under reduced pressure to provide the crude product. The crude material was dissolved in a minimal amount of dichloromethane and purified by flash chromatography on SiO2 (15-30% EtOAc-hexanes, elution was observed at 285 nm and 228 nm). The product 2 was a colorless solid that existed as a mixture of rotamers in solution at room temperature 94%: [α]26D -69.0 (c 0.21, CHCl3); 1H NMR (CDCl3) δ 0.81-1.02 (m, 6H), 1.06-1.17 (m, 6H), 1.23-1.38 (m, 9H), 2.51-2.63 (m, 1H), 2.64-2.77 (m, 1H), 3.20-3.29 (m, 0.6H), 3.32-3.41 (m, 0.4H), 3.51-3.58 (m, 1H), 3.62-3.70 (m, 6H), 3.70-3.76 (m, 0.4H), 3.91-4.01 (m, 0.6H), 4.65-4.82 (m, 1H), 4.83-4.98 (m, 1H), 5.71 (apparent d, J=5.7 Hz, 0.6H), 5.78 (apparent d, J=7.9 Hz, 0.4H), 6.42-6.49 (m, 1H), 6.77 (s, 0.6H), 6.81 (s, 0.4H); 13C NMR (CDCl3) δ 21.02, 21.09, 21.18, 21.32, 27.24, 27.95, 28.02, 37.60, 39.34, 52.11, 52.83, 55.48, 55.52, 59.28, 60.08, 68.58, 68.76, 68.82, 79.46, 80.03, 110.09, 110.73, 111.13, 126.11, 126.18, 126.37, 127.07, 146.81, 146.87, 147.93, 153.86, 154.30, 166.29, 166.78, 166.94, 167.06. |
| 94% |
|
The dihydroisoquinoline 1 (1.0 eq.) and Boc anhydride (1.5 eq.) were dissolved in CH2Cl2 at room temperature to provide a 1.5 M solution with respect to the dihydroisoquinoline. The mixture was allowed to stir for 30 min. Following the allotted time, the reaction mixture was cooled to 0 C. and then diisopropylmalonate (1.5 eq.) followed by a pre-chilled solution of the Pd catalyst (0.008 eq.) in dichloromethane were added successively to the reaction mixture to provide a final reaction concentration of 0.84 M with respect to the starting dihydroisoquinoline. The reaction mixture was allowed to continue stirring at 2.5 C. for 15 h. Following this time EtOAc and brine were added to the reaction mixture. The aqueous layer was extracted with three portions of EtOAc and the combined organic layers were dried (Na2SO4), filtered, and concentrated under reduced pressure to provide the crude product. The crude material was dissolved in a minimal amount of dichloromethane and purified by flash chromatography on SiO2 (15-30% EtOAc-hexanes, elution was observed at 285 nm and 228 nm). The product 2 was a colorless solid that existed as a mixture of rotamers in solution at room temperature 94%: [α]26D -69.0 (c 0.21, CHCl3); 1H NMR (CDCl3) δ 0.81-1.02 (m, 6H), 1.06-1.17 (m, 6H), 1.23-1.38 (m, 9H), 2.51-2.63 (m, 1H), 2.64-2.77 (m, 1H), 3.20-3.29 (m, 0.6H), 3.32-3.41 (m, 0.4H), 3.51-3.58 (m, 1H), 3.62-3.70 (m, 6H), 3.70-3.76 (m, 0.4H), 3.91-4.01 (m, 0.6H), 4.65-4.82 (m, 1H), 4.83-4.98 (m, 1H), 5.71 (apparent d, J=5.7 Hz, 0.6H), 5.78 (apparent d, J=7.9 Hz, 0.4H), 6.42-6.49 (m, 1H), 6.77 (s, 0.6H), 6.81 (s, 0.4H); 13C NMR (CDCl3) δ 21.02, 21.09, 21.18, 21.32, 27.24, 27.95, 28.02, 37.60, 39.34, 52.11, 52.83, 55.48, 55.52, 59.28, 60.08, 68.58, 68.76, 68.82, 79.46, 80.03, 110.09, 110.73, 111.13, 126.11, 126.18, 126.37, 127.07, 146.81, 146.87, 147.93, 153.86, 154.30, 166.29, 166.78, 166.94, 167.06. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping