| 52% |
|
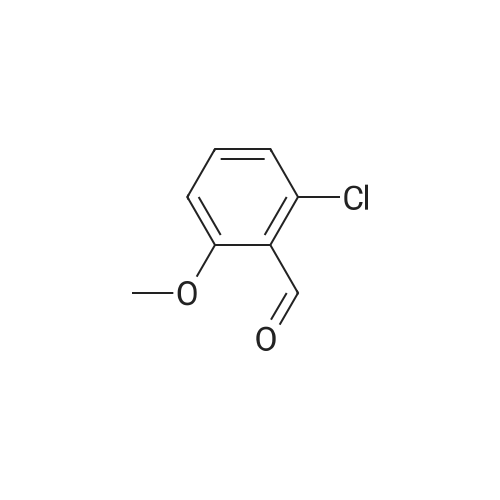
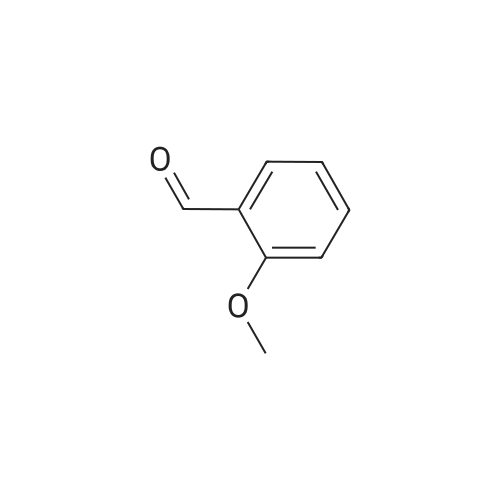
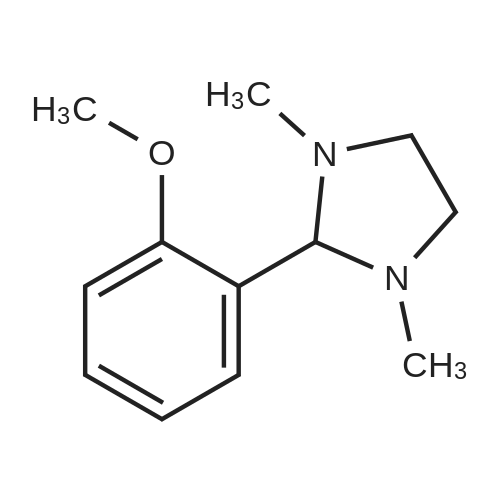
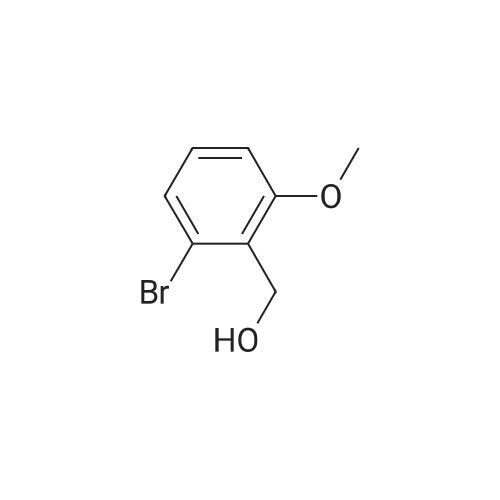
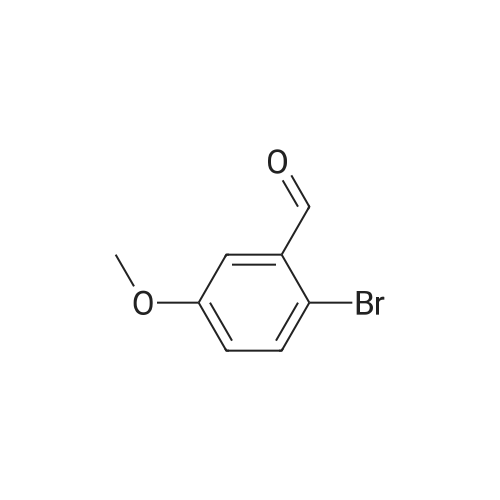
o-Anisaldehyde (10.0 g, 73.4 mmol, 1.0 equiv) was dissolved in EtOH (150 mL) at 25 CC, MAT-dimemylethylenediamine (8.70 mL, 80.8 mmol, 1.1 equiv) was added, and the reaction contents were stirred at 25 C for 24 h before being filtered through a pad of MgS04 and concentrated to afford the desired imidazolidine (15.0 g, 99% yield) as a white solid. Without any additional purification, this material (15.0 g, 72.8 mmol, 1.0 equiv) was dissolved in Et20 (250 mL) and cooled to -40 C. f-BuLi (1.7 M in pentane. 100 mL 170 mmol, 2.34 equiv) was then added dropwise over 1 h at -40 C. Upon completion, the resultant orange reaction contents were warmed slowly to -20 C. stirred for an additional 7 h, and then transferred by cannula over 5 min into a flask containing (CBrCl2)2 (55.3 g, 170 mmol, 2.34 equiv) in Et20 (250 mL) at 0 C. The reaction contents were then stirred for 12 h, during which time they were warmed to 25 C; upon completion, the solution was recooled to 0 C and 1 M HCI (500 mL) was added slowly. The resultant solution was stirred for 1 h at 0 C, quickly warmed to 25 C, and then quenched by the addition of water (500 mL). The reaction contents were then extracted with EtOAc (3 x 250 mL), and the combined organic extracts were washed with water (500 mL) and brine (250 mL). dried (MgSO-i), and 73 concentrated.'23' The resultant crude yellow solid was purified by flash column chromatography (silica gel, hexanes EtOAc, 9/1) to give the desired brominated product 28 (8.12 g, 52% yield) as a white solid. This material (8.12 g, 37.8 mmol, 1.0 equiv) was suspended in MeOH (100 mL) at 25 C and cooled to 0 C. NaBHj (2.88g , 75.6 mmol, 2.0 equiv) was added portionwise and the reaction contents were stirred for 1 h at 0 C. Upon completion, the reaction contents were quenched with water (100 mL) and concentrated. The reaction contents were redissolved in EtOAc ( 100 mL), poured into water (100 mL), and extracted with EtOAc (3 x 50 mL). The combined organic extracts were washed with water ( 150 mL) and brine (50 mL), dried (MgSO- , and concentrated to afford the desired alcohol (7.83 g, 96%) as a white solid. Pressing forward without any additional purification, this newly prepared material (7.83 g, 36.1 mmol, 1.0 equiv) was dissolved in EtjO (180 mL) and pyridine (0.437 mL, 5.41 mmol, 0.15 equiv) and PBr^ (3.41 mL, 36.1 mmol, 1.0 equiv) were added sequentially at 25 C. The reaction contents were then stirred for 4 h at 25 C. Upon completion, the reaction contents were quenched by the addition of water (100 mL), poured into water ( 100 ml), and extracted with EtOAc (3 x 150 mL). The combined organic extracts were washed with water (200 mL) and brine (100 mL), dried (MgS04), and concentrated to give the desired bromide (10.0 g, 99%) as a white solid. [Note: This product quickly decomposes on standing once it is neat and should be carried forward immediately. | Finally, KHMDS (0.5 M in toluene, 129 mL, 64.5 mmol, 1.8 equiv) was added to a solution of diethyl phosphite (9.19 mL, 71.4 mmol, 2.0 equiv) in THF (100 mL) at 0 C and stirred for 15 min. To this solution was added dropwise a solution of the freshly prepared bromide (10.0 g, 35.7 mmol, 1.0 equiv) dissolved in THF (100 mL), and the reaction contents were stirred for 12 h with slow warming to 25 C. Upon completion, the reaction contents were quenched with saturated NH4CI (150 mL), poured into water (150 mL), and extracted with EtOAc (3 x 150 mL). The combined organic extracts were washed with water (100 mL) and brine (100 mL), dried (MgS04), and concentrated to give the phosphonate 31 (10.79 g, 90%) as a colorless oil. 31: R/ = 0.21 (silica gel, EtOAc); IR (film) vmax 2981, 1589, 1572, 1466, 1435, 1267, 1082, 965, 864, 771 ; NMR (400 MHz, CDCI3) delta 7.18 (d, / = 8.0 Hz, 1 H), 7.07 (app dt, J = 8.0, 2.4 Hz, 1 H), 6.81 (d, J = 8.4 Hz, 1 H), 4.05 (dq, J = 7.2, 7.2 Hz, 4 H), 3.85 (s, 3 H), 3.50 (d, J = 22.0 Hz, 2 H), 1.26 (t, J = 7.2 Hz, 6 H); l3C NMR (75 MHz, CDCI3) delta 158.4 (d, J = 5.4 Hz). 128.6 (d, J = 3.8 Hz), 125.8 (d, J = 7.5 Hz), 125.0 (d, J = 3.5 Hz), 121.6 (d, J = 10.6 Hz), 109.4 (d, J = 3.4 Hz), 61.9 (d, J = 6.5 Hz), 55.9, 28.3 (d, J = 139.0 Hz), 16.3 (d, J = 6.4 Hz); HRMS (MALDI-FTMS) calcd for Ci2H|9BrP04+ [M + H*] 337.0204, found 337.0189. 74 |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping