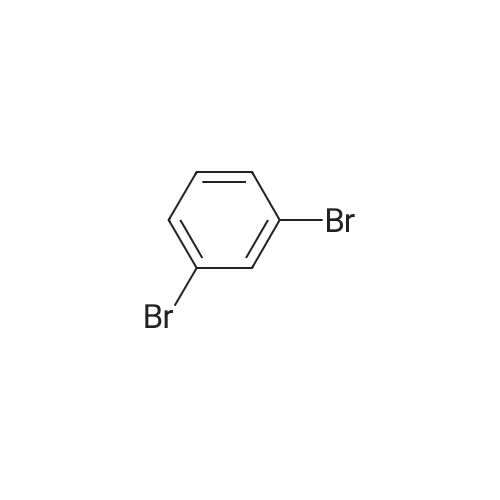
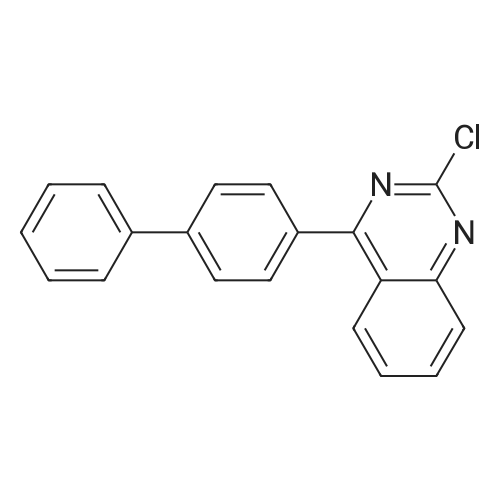
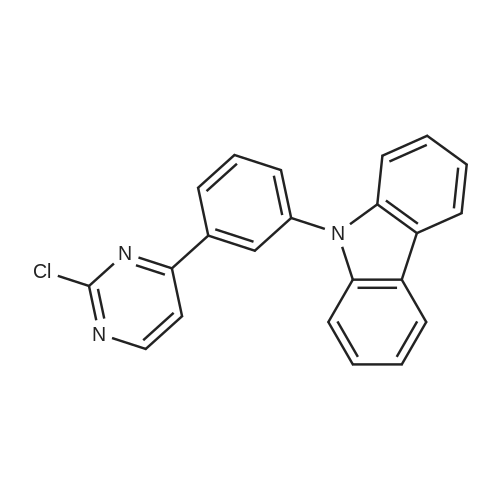
| 53% |
With di-tert-butyl(methyl)phosphonium tetrafluoroborate salt; palladium diacetate; In N,N-dimethyl acetamide; for 21h;Reflux; |
After mixing compound 1-1 (46 g, 143.8 mmol), Pd(OAc)2 (968 mg, 4.3 mmol), di-t-butyl(methyl)phosphoniumtetrafluoroborate (2 g, 4.31 mmol) and DMAc (200 mL), the reaction mixture was stirred for 21 hours under reflux. After terminating the reaction, the reaction mixture was filtered, and the filtered cake was washed with dichloromethane. The obtained organic layer was washed with purified water and dried with MgSO4, was concentrated under reduced pressure. And then, the crude oil was filtered through silica gel, and the remaining solution was concentrated under reduced pressure to obtain compound 1-2 (17 g, 53 %). |
| 47% |
With di-tert-butyl(methyl)phosphonium tetrafluoroborate salt; palladium diacetate; potassium carbonate; In N,N-dimethyl acetamide; at 200℃; for 12h; |
After mixing compound 1-1 (32 g, 0.1 mol), Pd(OAc)2 (1.1 g, 0.005 mol), di-t-butyl(methyl)phosphoniumtetrafluoroborate (2.48 g, 0.01 mol), K2CO3 (42 g, 0.30 mol) and DMA (550 mL), the reaction mixture was stirred for 12 hours at 200C. After terminating the reaction, the reaction mixture was extracted with EA. The obtained organic layer was dried with MgSO4, was filtered, was distillated under reduced pressure to remove the solvent, and was filtered through column to obtain compound 1-2 (14 g, 47 %). |
| 47% |
With di-tert-butyl(methyl)phosphonium tetrafluoroborate salt; palladium diacetate; potassium carbonate; at 200℃; for 12h; |
After mixing compound C-8-1 (32 g, 0.1 mol), Pd(OAc)2 (1.1 g, 0.005 mol), di-t-butylmethylphosphine·HBF4 (2.48 g, 0.01 mol), K2CO3 (42 g, 0.30 mol), and dimethyl amide (DMA) (550 mL), the mixture was stirred for 12 hours at 200C. After completing the reaction, the mixture was extracted with EA, and then the organic layer was dried with MgSO4. After filtering the obtained product, the solvent was removed under reduced pressure, and then the remaining product was separated with a column to obtain, white solid, compound C-8-2 (14 g, 47 %). |
| 47% |
With di-tert-butyl(methyl)phosphonium tetrafluoroborate salt; palladium diacetate; potassium carbonate; In N,N-dimethyl acetamide; at 200℃; for 12h; |
Compound 17-1 (32g, 0.1mol), Pd(OAc)2, (1.1g, 0.005mol), di-tert-butylmethylphosphine.HBF4 (2.48g, 0.01mol), K2CO3 (42g, 0.30mol), and DMA(550ml) were added, and then stirred at 200 for 12 hours. After completion of the reaction, extraction with ethyl acetate was performed on the resultant material, and then the organic layer is dried over MgSO4, followed by filtration. The solvent is removed under reduced pressure, followed by column chromatography, thereby obtaining Compound 17-2 (14g, 47 %). |
| 43% |
With palladium diacetate; sodium carbonate; tricyclohexylphosphine tetrafluoroborate; In N,N-dimethyl acetamide; for 5h;Reflux; |
In a flask was added Intermediate 18-3 (40g, 0,125mol), sodium carbonate (40g, 0.38mol), palladium acetate (0.5g, 2.2mmol), tricyclohexylphosphine tetrafluoroborate (1.6g, 4.4 mmol) and dimethylacetamide (0.5L), was heated at reflux for 5 hours, cooled, the solvent was evaporated under reduced partially removed, cooled, added to water, extracted with ethyl acetate, dried, purified by column to give the product 15g, yield 43%. |
| 43% |
With palladium diacetate; sodium carbonate; tricyclohexylphosphine tetrafluoroborate; In N,N-dimethyl acetamide; for 5h;Reflux; |
In a flask, intermediate 2-1 (40 g, 0.125 mol), sodium carbonate (40 g, 0.38 mol), palladium acetate (0.5 g,2.2 mmol), tricyclohexylphosphine tetrafluoroborate (1.6 g, 4.4 mmol) and dimethylacetamide (0.5 L) were heated at reflux for 5 hours, cooled, steamed to remove some of the solvent, cooled, and added to water. Ethyl acetate extraction, drying, and column purification gave 15 g of product in 43% yield. |
| 43% |
With palladium diacetate; sodium carbonate; tricyclohexylphosphine tetrafluoroborate; In N,N-dimethyl acetamide; for 5h;Reflux; |
In the flask,Intermediate 1-1 (40 g, 0.125 mol) was added,Sodium carbonate (40g, 0.38mol),Palladium acetate (0.5g, 2.2mmol),Tricyclohexylphosphine tetrafluoroborate (1.6 g, 4.4 mmol)And dimethylacetamide (0.5L),Heat reflux for 5 hours, cool,Steam distillation to remove some of the solvent,Cool, add water,Extract with ethyl acetate, dry,After column purification gives 15g product,Yield 43%. |
| 42% |
With di-tert-butyl(methyl)phosphonium tetrafluoroborate salt; palladium diacetate; In N,N-dimethyl acetamide; for 24h;Reflux; |
To a solution of compound 9-1 (2.5 g, 7.8 mmol), di-tert-butylmethylphosphonium tetrafluoroborate ((t-Bu) 2PMeHBF4, 0.057 g, 0.23 mmol), Pd (OAc) 2 (0.05 g, 0.23 mmol) was dissolved in dimethylacetamide (DMAC, 80 ml) and refluxed with stirring for 24 hours. After cooling to room temperature, the mixture was concentrated under reduced pressure and extracted with methylene chloride and water. The extract was subjected to column chromatography using toluene: Hx to obtain Compound 9-2 (0.9 g, 42%). |
| 36% |
With sodium carbonate;palladium diacetate; tricyclohexylphosphine tetrafluoroborate; In ISOPROPYLAMIDE; at 190℃; for 3h; |
Compound 6-1 (70g, 218mmol), Pd(OAc)2 (2.4g, 11mmol), PCy3HBF4 (8g, 22mmol), Na2CO3 (70g, 654mmol) and DMA 1.2L were mixed and stirred at 190C for 3 hours. After termination of the reaction, the mixture was extracted with EA 1L, and the obtained organic layer was washed with distilled water 200mL and dried with anhydrous MgSO4, and the organic solvent was removed under reduced pressure. The obtained solid was separated using silica gel column chromatography and recrystallization, yielding Compound 6-2 (22g, 36%). |
| 36% |
With sodium carbonate; tricyclohexylphosphine tetrafluoroborate;palladium diacetate; In ISOPROPYLAMIDE; at 190℃; for 3h; |
Compound 12-1 (70g, 218mmol), Pd(OAc)2 (2.4g, 11mmol,) tricyclohexylphosphine tetrafluoroborate (8g, 22mmol), Na2CO3 (70g, 654mmol) and DMA (1.2L) were mixed, and stirred at 190C for 3 hours. After stirring, the reaction mixture was cooled to room temperature and extracted with EA. Column separation was conducted on the solid product, yielding Compound 12-2 (22g, 36%). |
| 36% |
With palladium diacetate; sodium carbonate; tricyclohexylphosphine tetrafluoroborate; In N,N-dimethyl acetamide; at 190℃; for 3h; |
After mixing compound 10-1 70 g (218 mmol), Pd(OAc)2 2.4 g (11 mmol), PCy3HBF4 8 g (22 mmol), Na2CO3 70 g (654 mmol), and dimethylacetamide (DMA) 1.2 L, the mixture was stirred at 190 C for 3 hours. After completing the reaction, the mixture was extracted with ethylacetate 1 L, the obtained organic layer was washed with distilled water 200 mL, and dried with anhydrous MgSO4. Then, the organic solvent was removed under reduced pressure. Then, the obtained solid was separated using silica gel column chromatography and recrystallization to obtain compound 10-2 22 g (36%). |
| 36% |
With palladium diacetate; sodium carbonate; tricyclohexylphosphine tetrafluoroborate; In N,N-dimethyl acetamide; at 190℃; for 3h; |
Compound 8-1 (70.0 g, 218.0 mmol), Pd(OAc)2 (2.4 g, 11.0 mmol), tricyclohexylphosphine tetrafluoroborate (PCy3HBF4) (8.0 g, 22.0 mmol), Na2CO3 (70.0 g, 654.0 mmol), and dimethylacetamide (DMA) (1.2 L) were mixed in a flask and stirred at 190C for 3 hrs. After completing the reaction, the mixture was extracted with EA (1.0 L), and the obtained organic layer was washed with distilled water (200.0 mL) and dried over anhydrous MgSO4. The organic solvent was removed under reduced pressure. After the separation of the obtained solid via column chromatography on silica gel and recrystallization, compound 8-2 (22.0 g, 36 %) was obtained. |
| 36% |
With palladium diacetate; sodium carbonate; tricyclohexylphosphine tetrafluoroborate; In N,N-dimethyl acetamide; at 190℃; for 3h; |
70 g (218 mmol) of compound 10-1, 2.4 g (11 mmol) of Pd (OAc) 2, 8 g (22 mmol) PCy3HBF4, 70 g (654 mmol) of Na 2 CO 3 and 1.2 L of dimethylacetamide (DMA) were mixed and stirred at 190 C. for 3 hours. After the reaction, the mixture was extracted with 1 L of ethyl acetate, the organic layer was washed with 200 mL of distilled water, dried over anhydrous magnesium sulfate, and the organic solvent was removed under reduced pressure. The obtained solid was separated by silica gel column chromatography and recrystallization to give 22 g (36%) of compound 10-2. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping