| 93% |
With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; In dichloromethane; at 20℃; for 24.0h; |
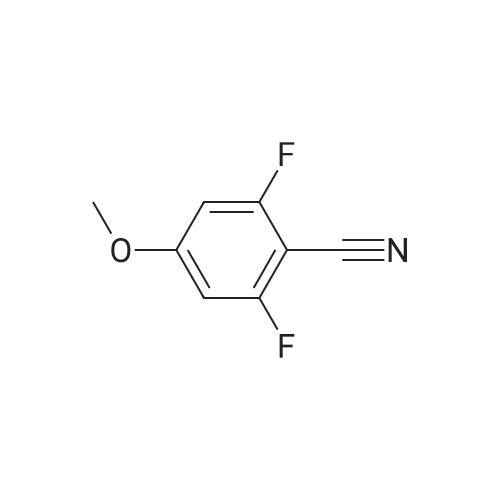
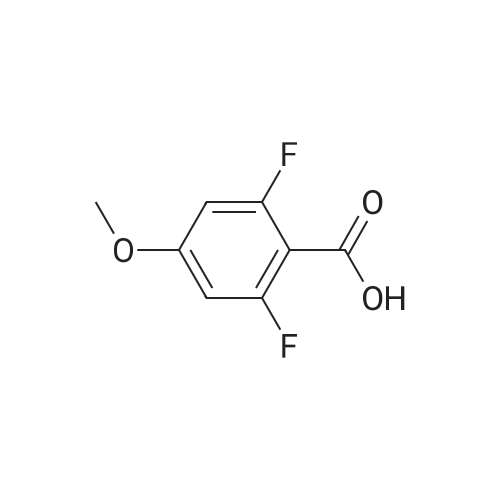
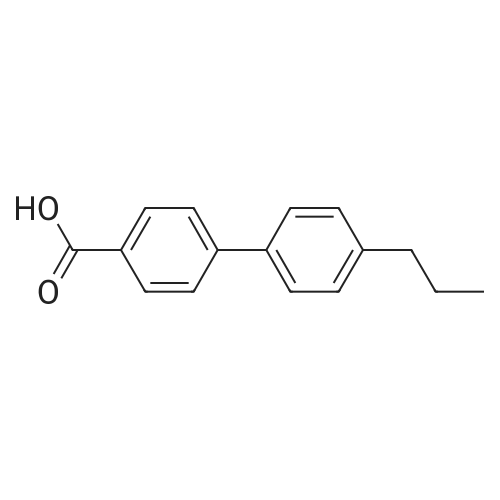
[Example 1] [0177] In this example, an example of synthesizing 4-(4-?-propylphenyl)benzoic acid 4-cyano-3,5-difluorophenyl (abbreviation: PPEP-3FCNF) represented by the structural formula (103) in Embodiment 1 will be described. [0178] (Synthesis method of 4-(4-?-propylphenyl)benzoic acid 4-cyano-3,5-difluorophenyl (abbreviation: PPEP-3FCNF))[0179] A synthetic scheme of PPEP-3FCNF (abbreviation) represented by structural formula (103) is shown in (B-1) below. [0180][0181] Into a 50-mL recovery flask were put 2.4 g (10 mmol) of <strong>[88038-94-2]4-(4-n-propylphenyl)benzoic acid</strong>, 1.6 g (10 mmol) of 2,6-difluoro-4-hydroxybenzonitrile, 0.18 g (1.5 mmol) of 4-dimethylaminopyridine, and 10 mL of dichloromethane, and stirring was performed. To this mixture, 2.2 g (11 mmol) of l-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochroride (EDC) was added, and stirring was performed in the air at room temperature for 24 hours. After predetermined time passed, water was added to the obtained mixture to extract an aqueous layer of this mixture with dichloromethane. The obtained extracted solution and an organic layer were combined and washed with a saturated aqueous solution of sodium hydrogen carbonate and saturated saline, and then, the mixture was dried with magnesium sulfate. The mixture was gravity filtered, and the obtained filtrate was condensed to give a yellow solid. This solid was purified by silica gel column chromatography (developing solvent: chloroform). The obtained fraction was concentrated to give a yellow solid. This solid was purified by high performance liquid chromatography (HPLC) (developing solvent: chloroform). The obtained fraction was concentrated to give 3.5 g of a white solid, which was a target substance, in a yield of 93 %.[0182] Further, 1.6 g of the obtained white solid was purified by distillation, whereby 1.5 g of a white solid, which was a target substance, was obtained in a yield of 94 %.[0183] This compound was identified by a nuclear magnetic resonance method (NMR) as <strong>[88038-94-2]4-(4-n-propylphenyl)benzoic acid</strong> 4-cyano-3,5-difluorophenyl (PPEP-3FCNF) which was a target substance.[0184] The 1H NMR data of the obtained substance (PPEP-3FCNF) is as follows. 1H NMR (CDC13, 300 MHz): δ (ppm) = 0.96 (t, 3H), 1.62-1.74 (m, 2H), 2.64 (t, 2H), 7.07 (d, 2H), 7.29 (d, 2H), 7.56 (d, 2H), 7.73 (d, 2H), 8.18 (d, 2H). FIGS. 8A to 8C are 1H NMR charts. Note that FIG. 8B is an enlarged chart showing the range of 6.5 ppm to 8.5 ppm in FIG. 8A. Note also that FIG. 8C is an enlarged chart showing the range of 0.0 ppm to 3.0 ppm in FIG. 8A. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping