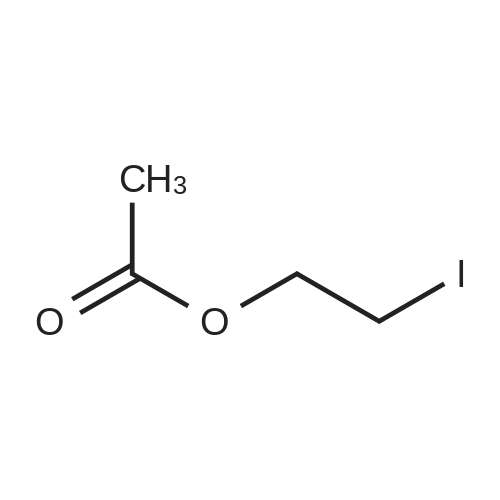
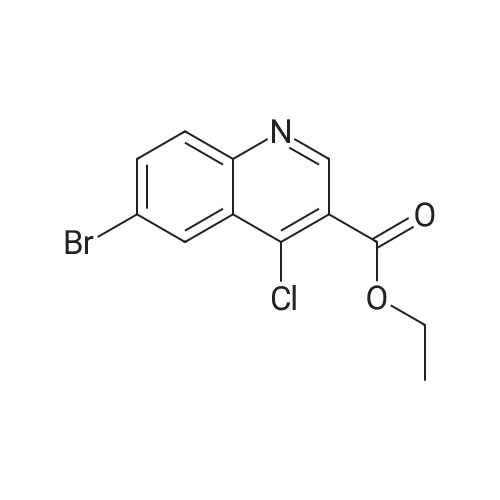
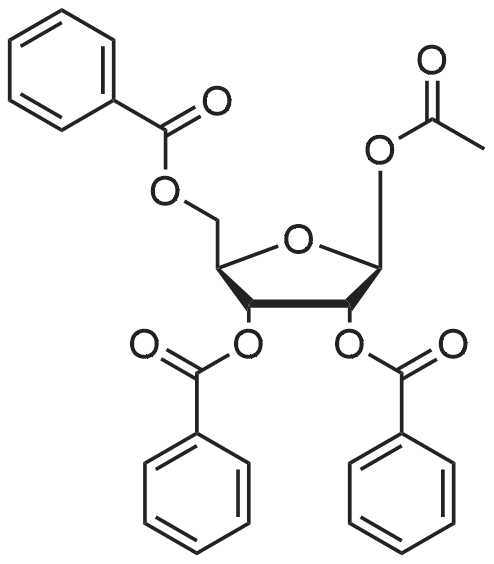
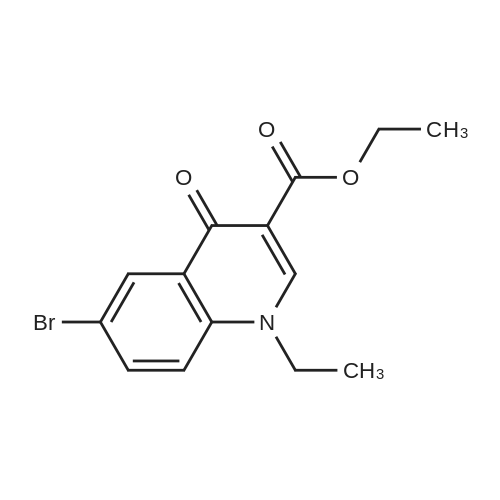
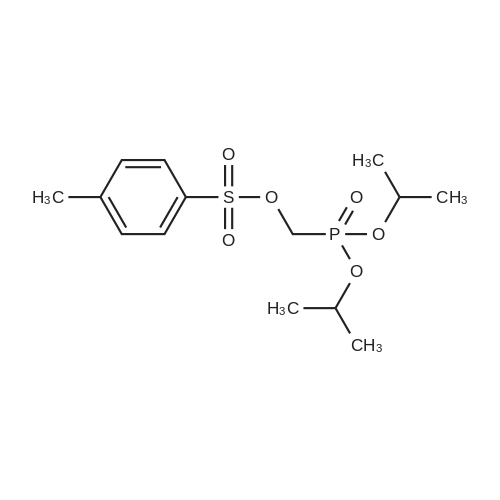
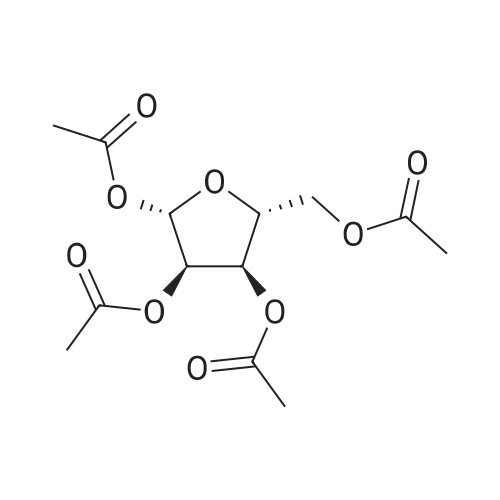
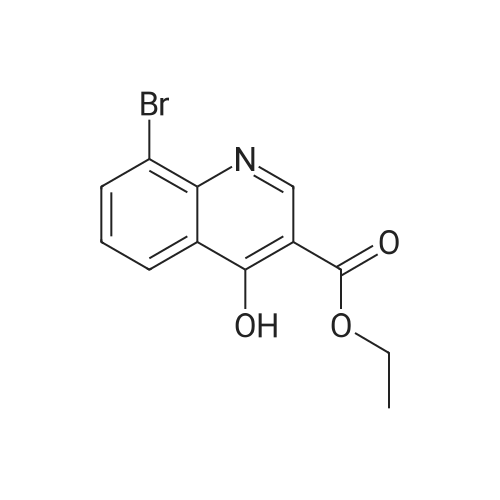
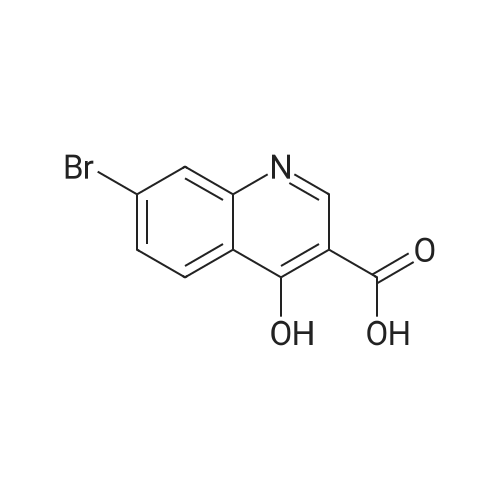
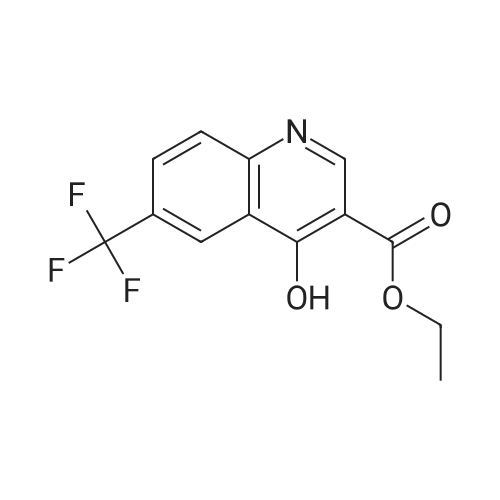
| 80% |
In diphenylether; at 220℃; for 30h; |
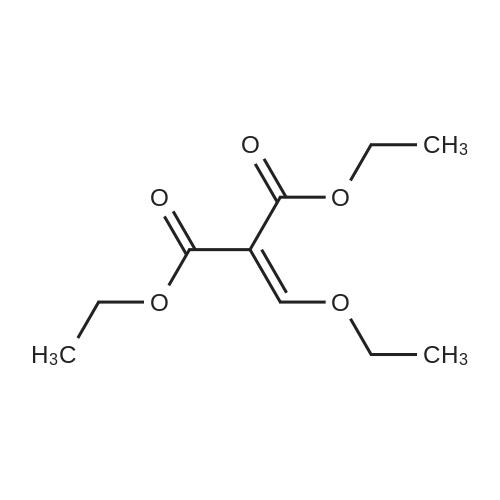
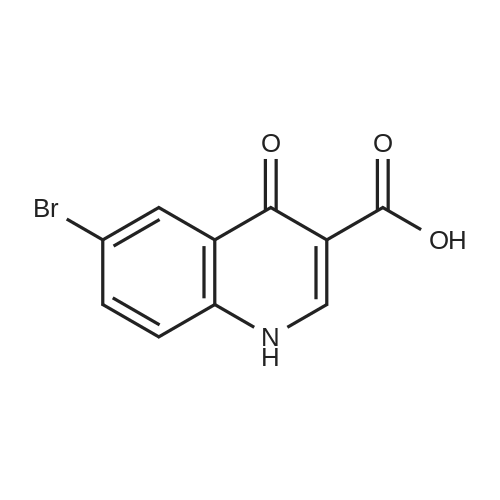
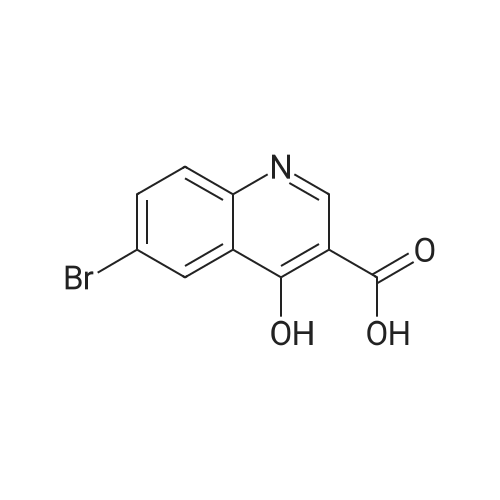
A suspension of 38.4 g of the product from the previous step (112mmol) in 200 ml diphenylether was heated up to 220 C internaltemperature and stirring was continued for 30 h. The reactionmixture was then cooled to room temperature and diluted with200 ml Et2O. The precipitate was isolated by filtration and washedsuccessively with EtOH and Et2O. The filter cake was dried in avacuum oven at 60 C overnight to yield 26.4 g (80%) of the titlecompound as off-white crystalline solid. ESI-MS m/z: 317.7[MNa] |
| 80% |
In diphenylether; at 220℃; for 30h; |
A suspension of 38.4 g of the product from the previous step (112mmol) in 200 ml diphenylether was heated up to 220 C internaltemperature and stirring was continued for 30 h. The reactionmixture was then cooled to room temperature and diluted with200 ml Et2O. The precipitate was isolated by filtration and washedsuccessively with EtOH and Et2O. The filter cake was dried in avacuum oven at 60 C overnight to yield 26.4 g (80%) of the titlecompound as off-white crystalline solid. ESI-MS m/z: 317.7[MNa] |
| 77.4% |
With trichlorophosphate; In water monomer; at 20 - 70℃; for 12h; |
General procedure: To a three-necked flask, 5 (300mg, 0.61mmol), PPA (1.5g) and POCl3 (2.5g) were added at room temperature. The mixture was heated to 70C for 12h and then cooled to room temperature. The mixture was treated with aqueous Na2CO3 solution to remove the remanent acid and filtered, and the solid was washed with water and dried to afford the product, which could be used in next step. m.p. 211-212C; 1H NMR (400MHz, DMSO-d6): δ 12.80 (s, 1H, br), 10.92 (s, 1H), 8.60 (s, 1H), 8.56 (d, 1H, J=2.0Hz), 8.02 (dd, 1H, J1=8.7Hz, J2=2.0Hz), 7.80 (d, 1H, J=8.7Hz), 7.50 (t, 1H, J=7.7Hz), 7.36-7.45 (m, 3H), 4.24 (q, 2H, J=7.2Hz), 1.29 (t, 3H, J=7.2Hz); ESI-MS (m/z): 441 (M+H)+ |
|
In diphenylether;Reflux; |
General procedure: The quinolone derivatives 1 were prepared by treating the appropriate aniline (100 mmol) with diethyl ethoxymethylenemalonate (100 mmol) under reflux in ethanol (5 mL) for 2-10 h to obtain the enamine derivatives that were then cyclized in refluxing diphenyl ether for 30 min-6 h [29]. These quinolones (13 mmol) were refluxed in thionyl chloride (20 mL), for 17 h, affording the corresponding chloro-derivatives 2a-g [22]. Reaction of 2a-g (4 mmol) with 2-hydrazinobenzothiazole (8 mmol) in toluene (30 mL), for 3 h, followed by a 2 h reflux in acetic acid gave the corresponding 2-(benzo[d]thiazol-2-yl)-8-substituted-2H-pyrazolo[4,3-c]quinolin-3(5H)-ones 3a-g as solids which were collected by filtration under vacuum, washed with water and subsequent purified by washing with hot ethyl alcohol. |
|
In diphenylether; for 1h;Reflux; |
General procedure: Substituted aniline 1 (0.01 mol) and diethylethoxy methylene malonate 2 (0.01 mol) weremixed and heated at 125-130 0C for 2 h. Ethanolwas evaporated off from the resulting mixture ofethyl anilinomethylene malonate 3. The crude solidwas filtered on sintered funnel, dried and recrystallized from n-hexane. The malonate 3 (0.01mol) was refluxed with diphenylether (50 mL) for 1h to give 1,4-dihydro-4-oxoquinoline-3-carboxylicacid ethyl ester 4. After 1 h the solution was cooledand the resulting precipitate was filtered off, washedwith n-hexane, and dried. The solid wasrecrystallized from DMF to give substituted-1,4-dihydro-4-oxoquinoline-3-carboxylic acid ethylester 4. |
|
In diphenylether; at 240℃; for 1h;Dean-Stark; |
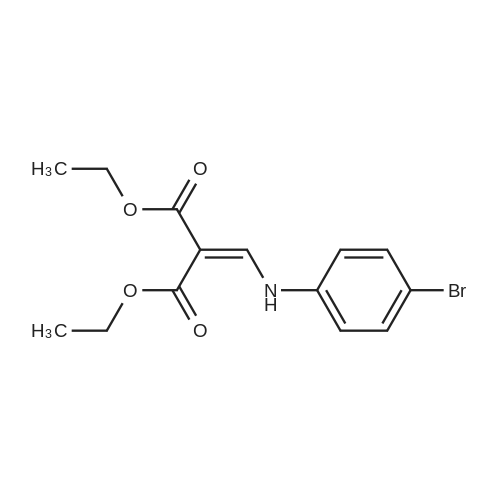
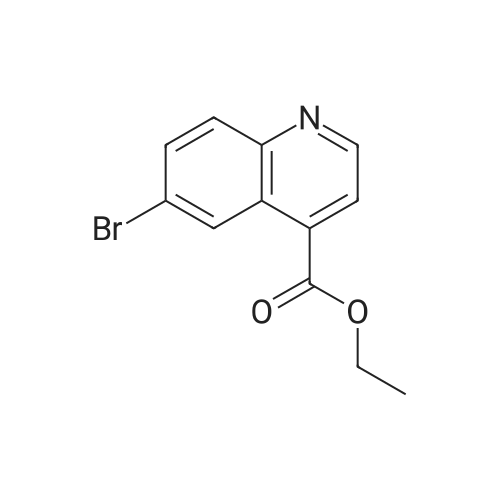
Diphenyl ether (870mL) was heated to 240C then diethyl 2-[[(4- bromophenyl)amino]methylidene]propanedioate (75g, 219.18mmol) added portionwise. The mixture was stirred at 240C for 60 minutes in a flask fitted with dean-stark apparatus. After cooling (25C) a crystallized solid was formed. The mixture was diluted with Et20 and the solid was collected by filtration, washed with Et20 and dried to afford the desired material (59.9g) as a beige crystallized solid, which was used without purification or characterisation. |
|
In diphenylether; at 260℃; for 9.5h; |
Diphenyl ether (100 g) was then added to the crude solid and the mixture was refluxed at 260C for 8 hours. After 1.5 hours a white solid started crushing out of solution in the reaction flask. T.l.c. analysis (ethyl acetate cyclohexane, 1: 4) showed the presence of one UV-active product (Rf 0.75) andcomplete consumption of the intermediate starting material 1 (Rf 0.49). Upon cooling of the reaction solution to room temperature, more ethyl 6-bromo-4-oxo-1,4- dihydroquinoline-3-carboxylate 2 crushed out of solution as a white powder (at 230C). The white solid was filtered, washed with toluene and dried in vacuo to afford ethyl 6-bromo-4-oxo- 1 ,4-dihydroquinoline-3 -carboxylate 2 (2.5 87g, 43.4%) asa pale yellow/white powder. M.p. 285-290C (decomposition: gas evolved); HRMS(El): found 294.983 14 [M] C12H10NO379Br requires 294.98386; found 296.98073[M] C12H10NO381Br requires 296.98 152; peak ratio: 49.5% : 50.5%; Vmax (Ge): 3421(br s, NH), 3149, 3088 (w, ArC-H), 2981 (m, alkyl C-H), 1694 (s, 2 xintramolecularly hydrogen-bonded C=O conjugated with C=C), 1615 (s, CCconjugated with C=O) cm’; Elemental Analysis: found C 48.36%, H 3.34%, N 4.64%; required C 48.67%, H 3.40%, N 4.73%. |
| 59.9 g |
In diphenylether; at 240℃; for 1h;Dean-Stark; Inert atmosphere; |
Diphenyl ether (870 mL) was heated to 240C then diethyl [(4- bromophenyl)amino]methylidene}propanedioate (75 g, 219.18 mmol) added portionwise. The mixture was stirred at 240C for 60 minutes in a flask fitted with Dean-Stark apparatus. After cooling ,the mixture was diluted with Et20 and the solid was collected by filtration, washed with Et20 and dried to afford ethyl 6-bromo-4-oxo-1H-quinoline-3-carboxylate (59.9g) as a beige crystallized solid, which was used without purification or characterisation. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping