| 74% |
|
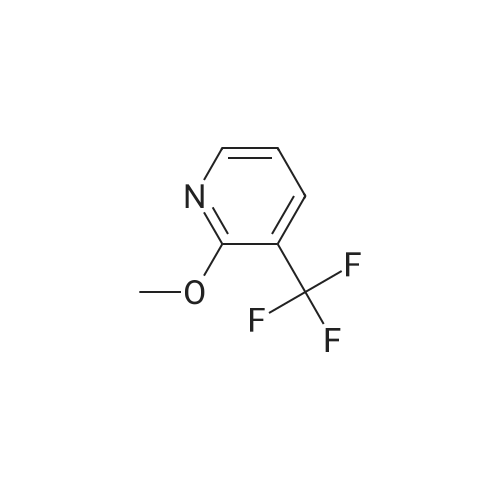
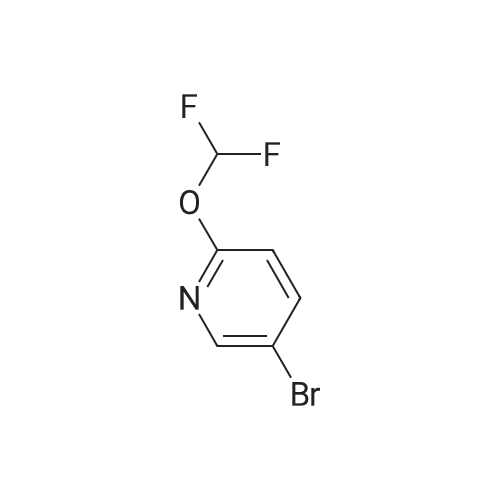
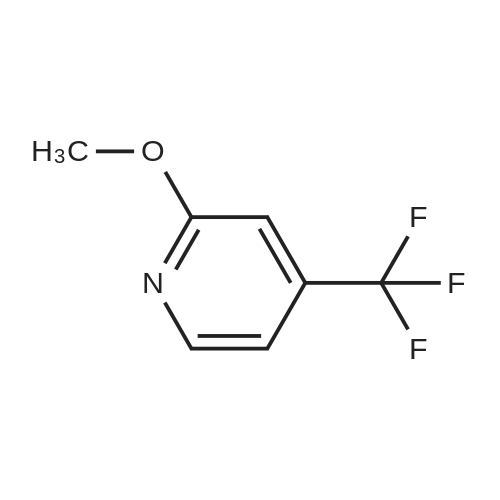
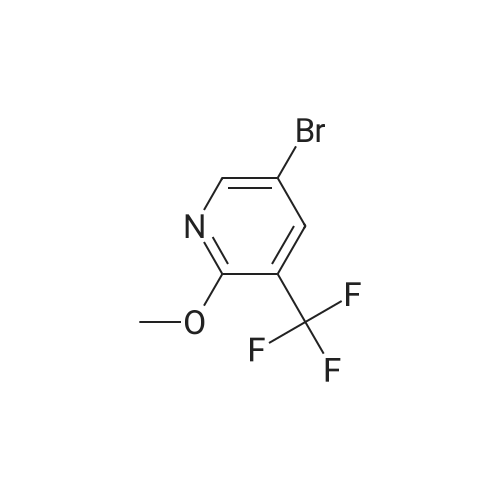
Intermediate 1 : 5-Bromo-<strong>[121643-44-5]2-methoxy-3-trifluoromethyl-pyridine</strong> To <strong>[121643-44-5]2-methoxy-3-(trifluoromethyl)pyridine</strong> (20.0 g, 1 13.0 mmol) and 1 ,3-dibromo-5,5- dimethylimidazolidine-2,4-dione (43.6 g, 152.0 mmol) was added TFA (80 mL) and the resulting mixture stirred at rt for 18h under argon. The TFA was removed in vacuo (50 mbar, 45C) and the residue suspended in tert-butyl methyl ether (200 mL). The resulting colourless solid was removed by filtration and washed with tert-butyl methyl ether (50 mL). The filtrate was concentrated in vacuo and suspended in EtOAc (50 mL) The insoluble colourless solid was removed by filtration and washed with EtOAc (50 mL).The filtrate was concentrated in vacuo, diluted with heptane/ tert-butyl methyl ether (5/1 , 20 mL) and the insoluble colourless solid was removed by filtration. The filtrate was purified by column chromatography on silica gel with heptane / EtOAc, 100/0 to 90/10. The crude product was filtered through a plug of NaHC03 (20g) and the filtrate evaporated in vacuo to give a golden oil (27.9 g). The oil was dissolved in heptanes (20 mL) and purified by filtered through a plug of silica gel (80 g), eluting with heptane to give 5-bromo-<strong>[121643-44-5]2-methoxy-3-(trifluoromethyl)pyridine</strong> as a colourless oil (22.5g, 74% yield). 1 H-NMR (400 MHz, DMSO-d6, 298 K): delta ppm 4.03 (s, 3H) 7.95 (d, 1 H) 8.4 (d, 1 H). |
| 74% |
With 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione; In trifluoroacetic acid; at 20℃; for 18h;Inert atmosphere; |
Intermediate 1 : 5-Bromo-<strong>[121643-44-5]2-methoxy-3-trifluoromethyl-pyridine</strong>To <strong>[121643-44-5]2-methoxy-3-(trifluoromethyl)pyridine</strong> (20.0 g, 1 13.0 mmol) and 1 ,3-dibromo-5,5- dimethylimidazolidine-2,4-dione (43.6 g, 152.0 mmol) was added TFA (80 mL) and the resulting mixture stirred at rt for 18h under argon. The TFA was removed in vacuo (50 mbar, 45C) and the residue suspended in tert-butyl methyl ether (200 mL). The resulting colourless solid was removed by filtration and washed with tert-butyl methyl ether (50 mL). The filtrate was concentrated in vacuo and suspended in EtOAc (50 mL) The insoluble colourless solid was removed by filtration and washed with EtOAc (50 mL).The filtrate was concentrated in vacuo, diluted with heptane/ tert-butyl methyl ether (5/1 , 20 mL) and the insoluble colourless solid was removed by filtration. The filtrate was purified by column chromatography on silica gel with heptane / EtOAc, 100/0 to 90/10. The crude product was filtered through a plug of NaHC03 (20g) and the filtrate evaporated in vacuo to give a golden oil (27.9 g). The oil was dissolved in heptanes (20 mL) and purified by filtered through a plug of silica gel (80 g), eluting with heptane to give 5-bromo-<strong>[121643-44-5]2-methoxy-3-(trifluoromethyl)pyridine</strong> as a colourless oil (22.5g, 74% yield). 1H-NMR (400 MHz, DMSO-d6, 298 K): delta ppm 4.03 (s, 3H) 7.95 (d, 1 H) 8.4 (d, 1 H). |
| 74% |
With 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione; trifluoroacetic acid; at 20℃; for 18h;Inert atmosphere; |
To <strong>[121643-44-5]2-methoxy-3-(trifluoromethyl)pyridine</strong> (20.0 g, 1 13.0 mmol) and 1 ,3-dibromo-5,5-dimethylimidazolidine-2,4-dione (43.6 g, 152.0 mmol) was added TFA (80 ml_) and the resulting mixture stirred at rt for 8h under argon. The TFA was removed in vacuo (50 mbar, 45C) and the residue suspended in tert-butyl methyl ether (200 ml_). The resultingcolourless solid was removed by filtration and washed with tert-butyl methyl ether (50 mL). The filtrate was concentrated in vacuo and suspended in EtOAc (50 mL) The insoluble colourless solid was removed by filtration and washed with EtOAc (50 mL).The filtrate was concentrated in vacuo, diluted with heptane/ tert-butyl methyl ether (5/1 , 20 mL) and the insoluble colourless solid was removed by filtration. The filtrate was purified by column chromatography on silica gel with heptane / EtOAc, 100/0 to 90/10. The crude product was filtered through a plug of NaHC03 (20g) and the filtrate evaporated in vacuo to give a golden oil (27.9 g). The oil was dissolved in heptanes (20 mL) and purified by filtered through a plug of silica gel (80 g), eluting with heptane to give 5-bromo-<strong>[121643-44-5]2-methoxy-3-(trifluoromethyl)pyridine</strong> as a colourless oil (22.5g, 74% yield).1H-NMR (400 MHz, DMSO-d6,298 K): delta ppm 4.03 (s, 3H) 7.95 (d, 1 H) 8.4 (d, 1 H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping