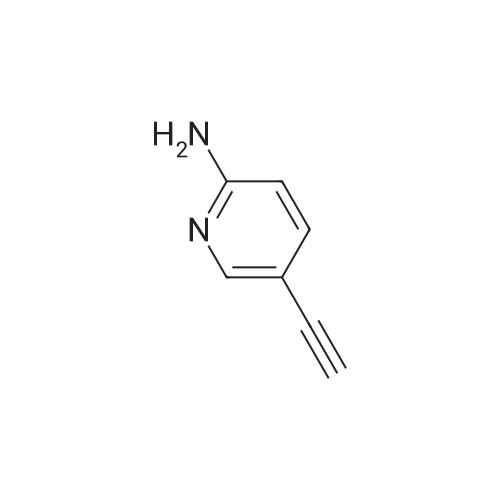
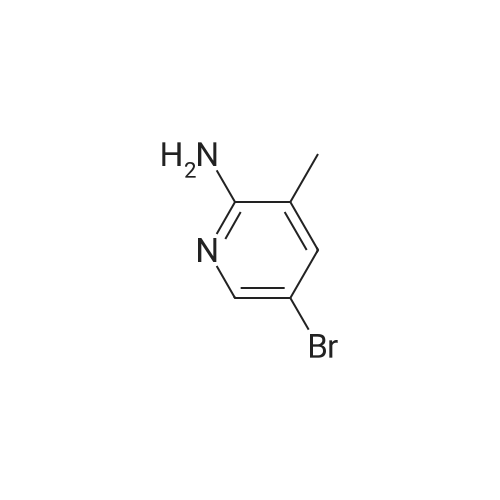
| 90% |
With potassium tert-butylate; In N,N-dimethyl-formamide; at 60 - 85℃;Large scale; |
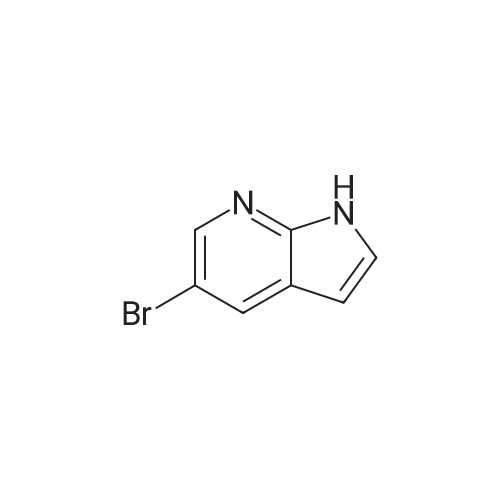
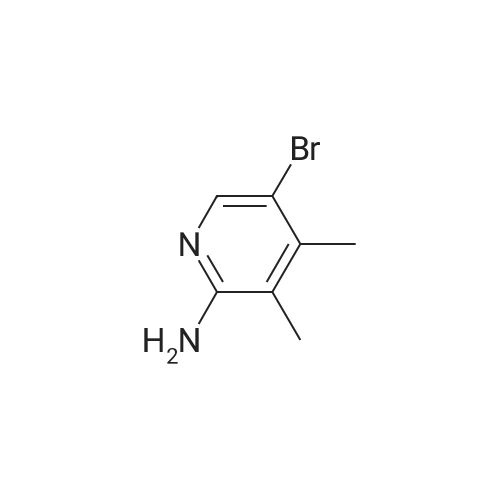
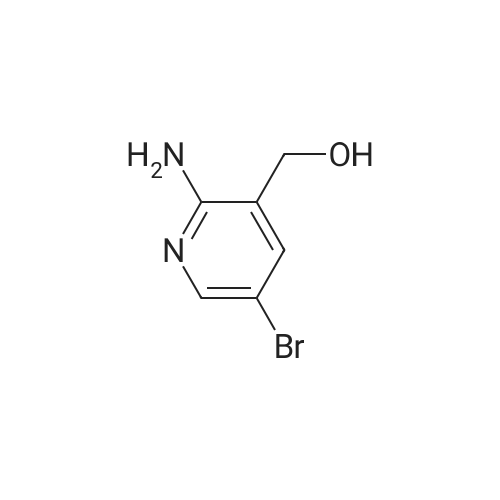
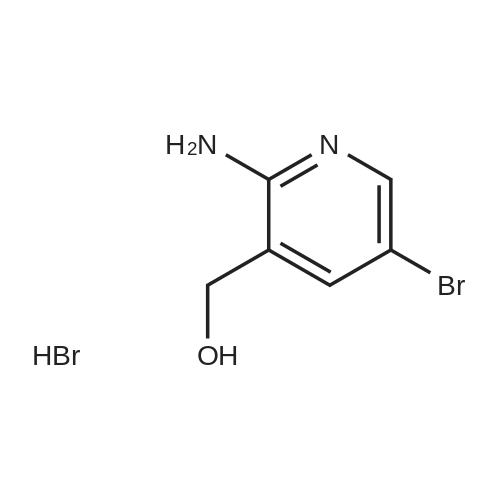
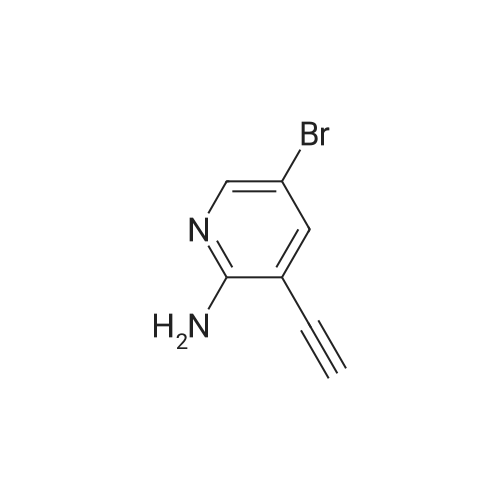
Add 150 kg of DMF to a 200 L reactor, slowly add 70 kg of potassium t-butoxide, stir to 60-70 C, and slowly add 50 kg of <strong>[1210838-82-6]5-bromo-3-ethynyl-2-aminopyridine</strong>. The temperature control is not higher than 80 C , plus complete the reaction incubated 80-85 C 2 ~ 3h, TLC monitoring completion of the reaction (PE / DCM = 1/1), cooled after completion of the reaction, the reaction system was slowly added to 400kg of ice water, cooled to 10 C was stirred for 2h, filtered off with suction (suction Should difficult, adding an appropriate amount of filter aid such as diatomaceous earth, etc.), to give a brown solid crude wet weight of about 75 kg (about 95% HPLC purity). The wet product was added to a 500 L reaction vessel, 300 kg of ethyl acetate (EA) was added, 5 kg of activated carbon was added, and the mixture was heated under reflux for 30 minutes, and then suction filtered. The filter cake was washed with an appropriate amount of EA, and then the filtrate and the washing liquid were combined, and the EA was evaporated to about 250 kg under reduced pressure. (Remaining about 50kg in the kettle), cooling to 0-5 C for 2h, suction filtration, filter cake washed with appropriate amount of cold EA, blasting at 60 C to obtain a light yellow solid product 5-bromo-7-azaindole 45kg, yield 90%, HPLC purity 99.3% |
|
|
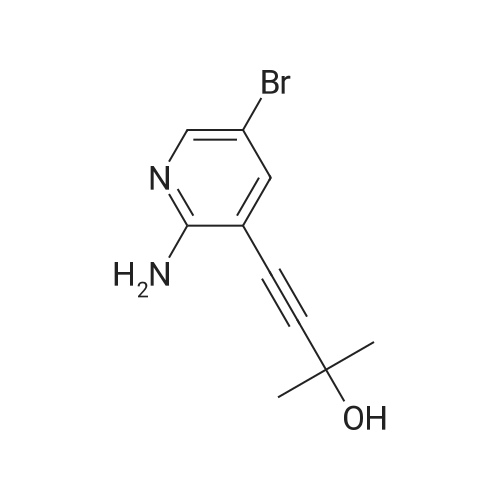
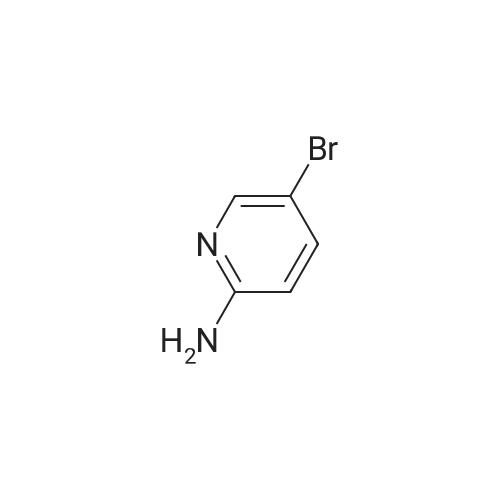
Example 7; a) Preparation of 5-bromo-7-azaindole (3 or Ia) from isolated 2-amino-5-bromo-3-iodopyridine; A suspension of 2-amino-5-bromo-3-iodopyridine (5.0 g, 16.7 mmol), bis-(triphenylphosphine)-palladium(II)-dichloride (43 mg, 0.061 mmol), copper(I)iodide (29.4 mg, 0.15 mmol) and triethylamine (2.21 g, 21.8 mmol) in dichloromethane (20 mL) was treated at 23 to 30 C. within 1 to 2 hours with a solution of 1,1-dimethyl-2-propyn-1-ol (1.85 g, 21.7 mmol) in dichloromethane (10 mL) and the resulting mixture was stirred at 25 C. for 4 hours. The mixture was diluted with dichloromethane (10 mL) and washed with water (2×25 mL). The organic phase was then treated with 1 M HCl (40 mL). The layers were separated and the organic layer was extracted with 1 M HCl (15 mL). The combined product containing aqueous layers were washed with dichloromethane (2×8 mL). The pH of the aqueous layer was adjusted to pH 7-9 by the drop wise addition of sodium hydroxide solution (28% in water). The resulting suspension was stirred at 20 C. over night and the crystals were then filtered off and washed with water (2×5 mL). The wet crystals were dissolved in N-methylpyrrolidone (50 mL) and treated within 2 hours at 60 C. and 50-100 mbar with an aqueous solution of lithium hydroxide (2.4 M, 32 mL). The resulting mixture was heated to 75 C. and stirred at this temperature and under reduced pressure (50-100 mbar) for 15-20 hours. Toluene (20 mL) and water (20 mL) were then added and the layers were separated. The aqueous layer was extracted with toluene (3×25 mL). The combined organic layers were washed with water (3×10 mL) and then concentrated to dryness. The residue was dissolved in N-methylpyrrolidone (50 mL) and treated at 60 C. with potassium tert.-butylate (3.52 g, 30.7 mmol). After stirring for 3 hours at 60 C., the mixture was cooled to ambient temperature and diluted with toluene (40 mL) and water (40 mL). The aqueous layer was separated and back extracted with toluene (3×50 mL). The combined toluene layers were washed with water (3×10 mL) and then concentrated to dryness. The residue was dissolved in a hot mixture of toluene and n-heptane (20 mL). The clear solution was cooled to -5 C. within 4 to 6 hours whereupon crystals precipitated. The suspension was stirred at -5 C. for 2-4 hours. The crystals were filtered off, washed with heptane and dried at 45 C./<30 mbars over night to afford 5-bromo-7-azaindole (2.05 g, 62% yield) as slightly yellow crystals with a purity of 99.6% (HPLC, area %). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping