| 91% |
With palladium on activated charcoal; hydrogen; In ethyl acetate; at 20℃; for 4h;Inert atmosphere; |
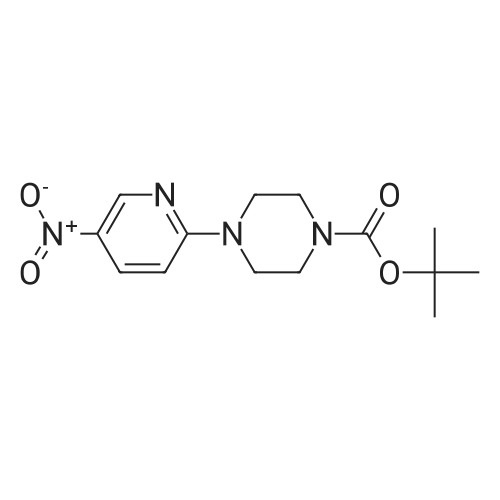
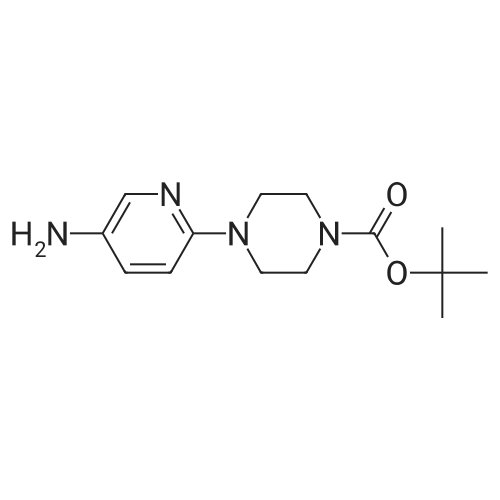
To a stirred solution of compound 46-3 (4-(5-Nitro-pyridin-2-yl)-piperazine-1-carboxylicacid tert-butyl ester) (1.7 g, 5.519 mmol) in EtOAc (50 mL) argon was purged for 10 min then PdC(800 mg) was added and the reaction was stirred under hydrogen atmosphere (balloon) for 4 hours. The reaction mixture was filtered through celite and concentrated under reduced pressureto afford compound 46-4 (1.4 g, 91%) as brown solid. LC MS: ES+ 278.9. |
| 90% |
With palladium 10% on activated carbon; hydrogen; In ethanol; ethyl acetate; at 70℃; under 750.075 Torr; for 0.5h;H-Cube; |
(General flow chemistry reduction method). Using 10% Pd/C as catalyst, a solution of compound 3b (1 mmol, 208 mg) in a 1:1 mixture of ethyl acetate: ethanol (30 mL) was pumped though the H-Cube. The pressure of the system was set to 1 bar, and the temperature to 70 C. After 30 minutes, all the reaction mixture had passed though the HCube. The fraction was analyzed using TLC, which showed complete conversion of the product, and the solvent was reduced to dryness, affording a dark red oil 171 mg (96%) yield. The CatCart was then washed with ethanol for approximately 10 minutes and the washings were discarded. |
| 68% |
With hydrogen;palladium 10% on activated carbon; In ethanol; at 20℃; for 5h; |
A suspension of <strong>[193902-78-2]4-(5-nitro-pyridin-2-yl)-piperazine-1-carboxylic acid tert-butyl ester</strong> (10.8 g) obtained in Example (38a) and 10% palladium-carbon catalyst (2.15 g) in ethanol (300 mL) was stirred at room temperature under a hydrogen atmosphere for five hours. The reaction mixture was filtered and concentrated. The residue was vigorously stirred in isopropyl ether, collected by filtration and dried under reduced pressure, and 6.59 g (68%) of the title compound was obtained as a pale pink solid. MS(FAB) m/z:279 (M + H)+. |
| 40% |
With iron; ammonium chloride; In ethanol; water; at 50℃; for 0.5h; |
To a stirred solution of 369 <strong>[193902-78-2]ter<strong>[193902-78-2]t-butyl 4-(5-nitropyridin-2-yl)piperazine-1-carboxylate</strong></strong> (2.9 g, 9.405 mmol, 1.0 eq) in 6 mL of 6 ethanol: 7 water (1:1) mixture were added 217 ammonium chloride (4.04 g, 75.24 mmol, 4 eq) and Fe(0) (2.10 g, 37.62 mmol, 4.0 eq). Reaction mixture was heated at 50 C. for 30 min. Progress of reaction was monitored by LCMS. Upon the consumption of starting material, the reaction mixture was filtered over celite and filtrate was concentrated under reduced pressure. The crude obtained was diluted with 50 mL of water and extracted with ethyl acetate (200 mL×2). Combined organic layer was washed with water and brine, dried over Na2SO4 and concentrated under reduced pressure. The crude was purified by flash chromatography using 0-2% 30 MeOH in 82 CH2Cl2 as eluents to obtain the desired product, 372 tert-butyl 4-(5-aminopyridin-2-yl)piperazine-1-carboxylate (1.072 g, 40%). (0463) LCMS: 279 [M+1]+ |
|
With hydrogen;palladium on activated charcoal; In ethanol; at 20℃; for 3h; |
A solution of 1-(terbutyloxycarbonyl)-4-(5-nitro-piridin-2-yl)-piperazine (4.4 g) in EtOH (150 mL) containing Pd/C (0.5 g) was hydrogenated at room temperature during 3 hours. The catalyst was filtered off and the filtrate was evaporated under reduced pressure. The titled compound was obtained as a brown oil (3.9 g). [0270] MS: m/z 279 (M+1). |
|
With hydrogen;palladium 10% on activated carbon; In methanol; ethyl acetate; at -10 - 45℃; under 3102.97 Torr; for 3h; |
[2- (N-T-BUTOXYCARBONYLPIPERAZINE)-5-NITRO] pyridine (82 g, 266.233 mmoles) was dissolved in 1: 1 mixture of methanol and ethylacetate [(1L).] This solution was cooled [TO-5 TO-10 C.] To this, 8.2 g of 10% palladium carbon was added and hydrogenated the reaction mixture at [45 C,] 60 psi for 3 hours. Filtered the reaction mixture through celite and washed the residue thoroughly with methanol. Concentrated the filtrate to dryness and dried under high vacuum to give the title compound (74 g). |
|
With hydrogen;palladium 10% on activated carbon; In methanol; ethyl acetate; at -10 - 45℃; under 3102.97 Torr; for 3h; |
2- (PIPERAZINE-N-T-BUTOXYCARBONYL)-5-NITRO pyridine (82 g, 0.266233 moles) was dissolved in 1: 1 mixture of methanol and ethylacetate (1L). This solution was cooled TO-5o TO-10 oC. To this, 8.2 g of 10% palladium carbon was added and hydrogenated the reaction mixture at 45 oC, 60 psi for 3 hours. Filtered the reaction mixture through celite and washed the residue thoroughly with methanol. Concentrated the filtrate to dryness and dried under high vacuum to give the title compound (74 g). |
|
With hydrogen;palladium 10% on activated carbon; In ethanol; under 3102.97 Torr; for 2h; |
4-(5-Nitro-pyridin-2-yl)-piperazine-l-carboxylic acid tert-butyl ester (5 g), 10% Pd/C (0.5 g) and ethanol (200 ml) were taken together in a hydrogenation flask and the reaction mixture was hydrogenated at 60 psi for 2 hrs. On completion, the reaction mixture was filtered through a celite bed and the ethanol was removed under reduced pressure to give the curde product. The compound was further purified by crystallization from 50% EtOAc/n-hexane (200 ml) to yield 4-(5-Amino-pyridin-2-yl)-piperazine-l-carboxylic acid tert-butyl ester. EPO <DP n="52"/>4-r5-d-Oxy-benzori,2,41triazin-3-ylamino)-pyridm-2-vn-piperazine-l-carboxylic acid tert-butyl ester |
|
With hydrogen;5% palladium over charcoal; In ethyl acetate; at 20℃; under 2327.23 Torr; for 3h; |
Method B:Step 1: tert-Butyl 4-(5-aminopyridin-2-yl)piperazine-l-carboxylate: To a stirred solution of tert-Butyl 4-(5-nitropyridin-2-yl)piperazine-l-carboxylate (20 g, 64.935 mmol) in EtOAc (200 ml) was added 5 % Pd/C (4 g) and the mixture was maintained under hydrogen pressure (45 psi) for 3 h at room temperature. The catalyst was then filtered off and the filtrate was concentrated under reduced pressure to afford 16.24 g of the amine as a light brown solid which was used as such for the next step. |
|
With palladium 10% on activated carbon; hydrogen; acetic acid; at 30℃; for 1h; |
29a (15.0 g of 54.0 mmol) was dissolved in 150 mL of acetic acid, and 1.6 g of 10% palladium carbon was added thereto, and hydrogenation was carried out at 30 C for 1 h.After completion of the reaction, the mixture was filtered under reduced pressure, and the filtrate was directly poured, and 4-fluorobenzaldehyde (8.9 g, 72.0 mmol) was added to the filtrate, stirred at room temperature for 1 hour, then cooled to about 10 C, and sodium borohydride was added.(4.13 g, 108.0 mmol), the reaction was kept for 30 min after the addition. After the completion of the reaction, the reaction mixture was poured into water, and the mixture was evaporated. Purification (PE: EA = 4:1, v/v) gave solid 29c.The yield in two steps was 65.0%; |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping