| 80.2% |
|
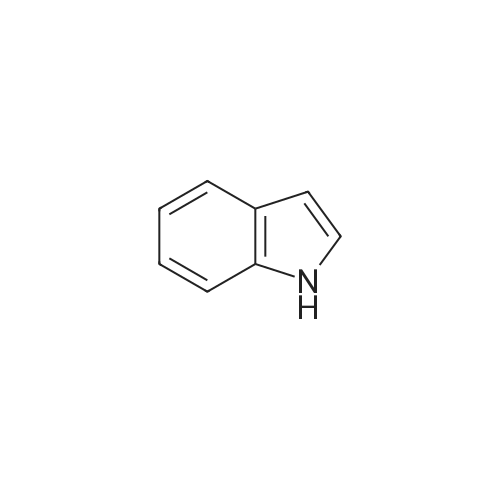
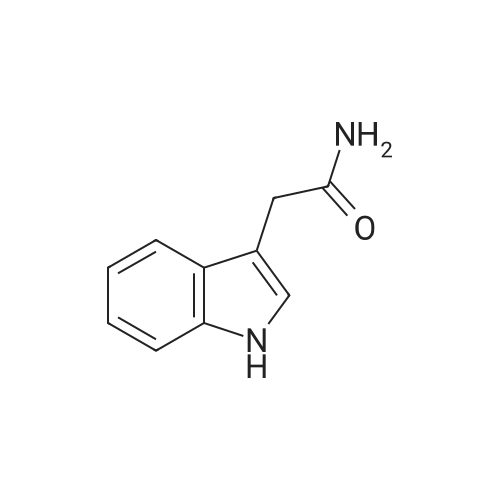
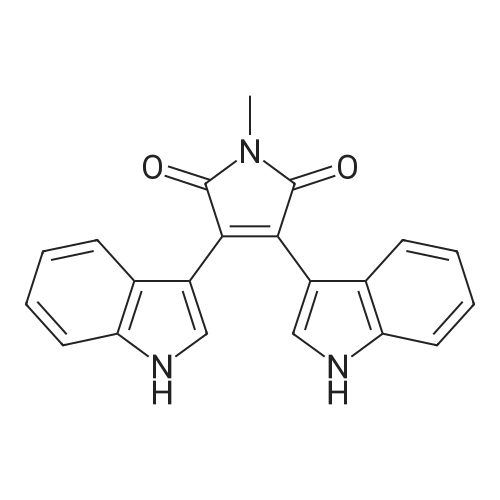
A. Chemical Examples; Example 1; 3,4-Di-(1 H-indol-3-yl)pyrrole-2,5-dione (Compound 1); Potassium tert-butoxide (0.976 g, 8.7 mmol) was added to a stirred suspension of (1 H-indol-3-yl)acetamide (0.5 g, 2.8 mmol) and methyl 3-indolylglyoxylate (0.650, 3.15 mmol) in anhydrous tetrahydrofuran (15 ml), under argon at -10C. After 15 minutes the resultant dark red solution was allowed to warm to 20C over 3.5 hours. Concentrated hydrochloric acid (2ml) was added with cooling, and the orange-red precipitate then dissolved in ethyl acetate by stirring overnight. The organic phase was washed with water and brine, dried (magnesium sulphate) and evaporated to give the title compound as red crystals (0.780 g, 80.2% yield), mp 234C. 1H-NMR (DMSO-d6, No./ppm) : 11.6 (br s, 1 H), 10.95 (br s, 1 H), 7.68 (s, 1 H), 7.38 (d, 1 H), 6.98 (t, 1 H), 6.8 (d, 1 H), 6.6 (t, 1 H). |
| 75% |
With potassium tert-butylate; In tetrahydrofuran; at 0 - 20℃; for 4.5h;Inert atmosphere; |
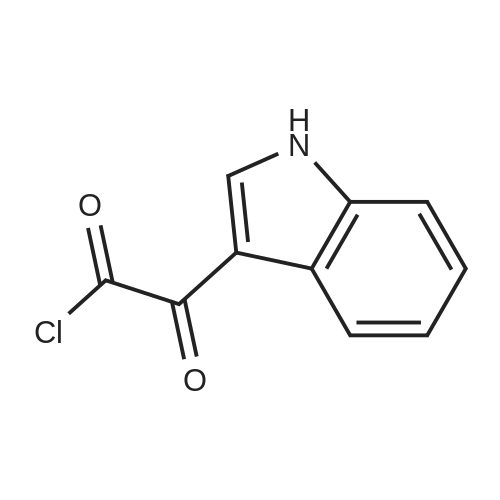
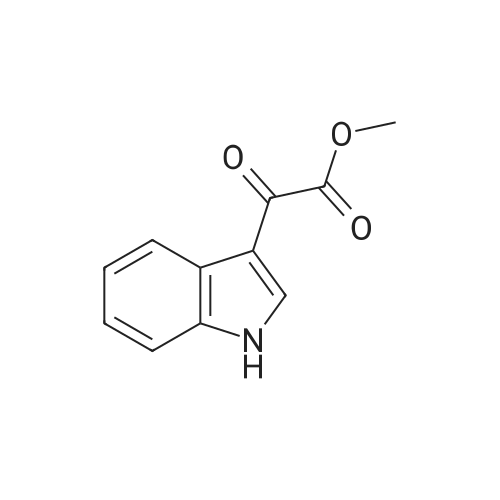
A three-necked flask equipped with a magnetic stirrer and two addition funnels was charged with indole (10.1 g, 0.086 mol) and 100 mL of diethyl ether. Oxalyl chloride (7.3 mL, 0.086 mol) was added dropwise to the solution at 0 C under nitrogen in 0.5 h. Yellow precipitate were formed and the reaction mixture was stirred for another 0.5 h. The reaction mixture was cooled to -70 C by dry-ice, then sodium methylate (25 % solution in methanol, 37.3 g, 0.173 mol) was added dropwise to the reaction mixture in 1 h. After that the reaction mixture was warmed to 0 C and 50 mL of water was added. The precipitate were filtered, washed with water several times, and then dried at 60 C under vacuum. The product of methyl indolyl-3- glyoxylate was obtained as a yellow powder and used without further purification. Yield 90 %. A three-necked flask equipped with a magnetic stirrer and an addition funnel was charged with 3-indoleacetamide (8.0 g, 0.046 mol), methyl indolyl-3-glyoxylate (10.0 g, 0.049 mol) and 80 mL of tetrahydrofuran. A solution of potassium tert-butoxide (15.2 g, 0.135 mol) in 130 mL of tetrahydrofuran was added dropwise to the reaction mixture at 0 C under nitrogen in 1.5 h. Then the reaction mixture was warmed to room temperature and stirred for 3 h. A solution of Hydrochloric acid (35 % in water, 64 mL) was added dropwise to the reaction mixture in 1 h. Then 200 mL of ethyl acetate and 100 mL of water were added and stirred for dissolving. The organic phase was separated, washed with water several times until neutral, and then washed with brine once, dried over anhydrous sodium sulfate. The sodium sulfate was filtered and the solution was concentrated. The product was crystallized by adding a 1:1 (v/v) mixture of ethyl acetate and n-hexane dropwise to the concentrated solution at 50~60 C. The pure product of 3,4-bisindolylmaleimide was obtained as a red crystal. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping