| 89.13% |
|
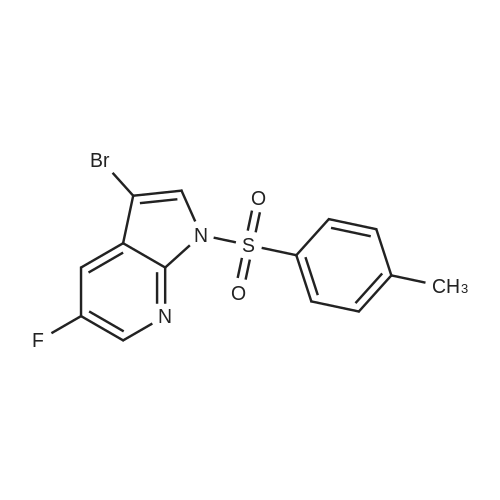
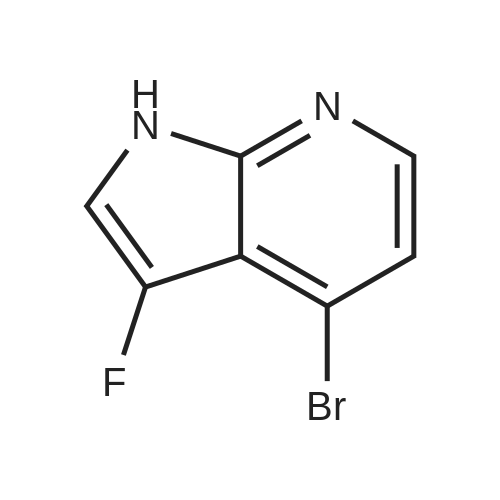
At 0 C., to a solution of the compound BB-3-5 (9.80 g, 45.58 mmol) in tetrahydrofuran (150.00 mL) was added sodium-hydrogen (2.19 g, 54.69 mmol, 60%). The reaction liquid was stirred at 15 C. for 30 minutes. P-toluene sulfonyl chloride (10.43 g, 54.69 mmol) was added into the reaction liquid, which was stirred at 15 C. for 12 hours. To the reaction liquid was added water (100 mL) dropwise, extracted with ethyl acetate (150 mL) for three times. The organic phases were combined, dried over anhydrous sodium sulfate, filtered, and concentrated at reduced pressure. The resulting crude product was purified over a silica gel chromatographic column (petroleum ether:ethylacetate=50:1 to 20:1) to give the compound BB-3-6 (15.00 g, 40.63 mmol, yield 89.13%). MS (ESI) m/z=370.7 [M+1]. |
| 83% |
|
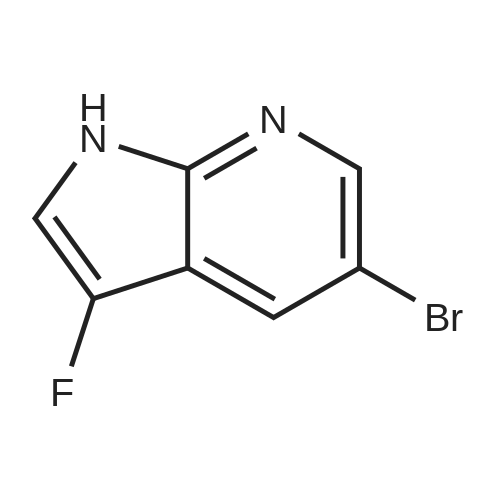
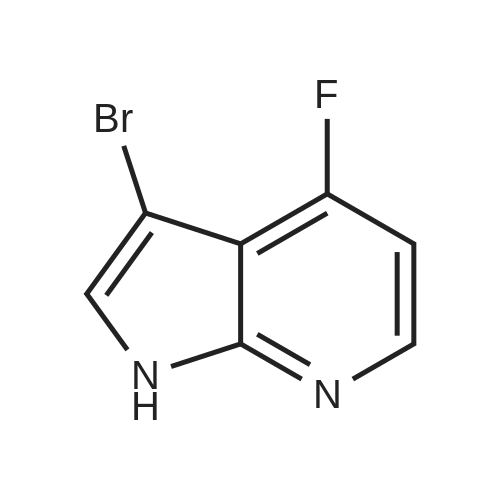
3-Bromo-5-fluoro-1H-pyrrolo[2,3-b]pyridine (0.74 g, 3.44 mmol) was dissolved in THF (5 mL)NaH (127 mg, 3.0 mmol) was added thereto at 0 C and stirred at this temperature for 30 minutes.Then add TsCl (458mg,2.4mmol),The mixture was transferred to room temperature and stirring was continued overnight.Add water (50mL) to quench the reaction, separate the liquid, and use the acetic acid in the aqueous phase.Ester extraction (50mL × 2),The combined organic phases were washed with brine (80 mL).Dry over anhydrous sodium sulfate, filter, and dilute the solvent under reduced pressure.The residue was purified by silica gel column chromatography(PE/EtOAc(v/v)=4/1)The title compound was obtained as a yellow solid(740 mg, 83%). |
| 76% |
|
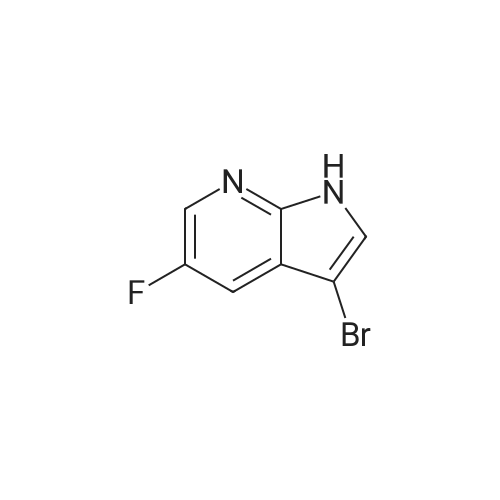
To a stirred suspension of NaH (1.3 g, 34 mmol) in DMF (30 mL) was added 3-bromo-5- fluoro 1H pyrrolo[2,3-b]pyridine 5 (4.6 g, 21 mmol) in DMF at 0 C. After 1h, a solution of p-TsCl (5.7 g, 30 mmol) in DMF (20 mL) was added slowly at the same temperature and stirred for 2 h. After completion of the reaction as indicated by TLC, the mixture was poured into ice cold water (200 mL) and filtered the precipitated solid and dried to afford 3-bromo-5-fluoro-1-tosyl-1H-pyrrolo[2,3-b]pyridine (6) (6.2 g, 16 mmol, 76 %) as an off-white solid. TLC system: 10% EtOAc in hexane Rf : 0.8 LCMS (ESI): m/z 369 [M+H]+ |
| 76% |
|
To a stirred suspension of NaH (1.3 g, 34 mmol) in DMF (30 mL) was added 3- bromo-5-fluoro 1H pyrrolo[2,3-b]pyridine 5 (4.6 g, 21 mmol) in DMF at 0 C. After 1h, a solution of p-TsCl (5.7 g, 30 mmol) in DMF (20 mL) was added slowly at the same temperature and stirred for 2 h. After completion of the reaction as indicated by TLC, the mixture was poured into ice cold water (200 mL) and filtered the precipitated solid and dried to afford 3-bromo-5-fluoro-1-tosyl-1H-pyrrolo[2,3-b]pyridine (6) (6.2 g, 16 mmol, 76 %) as an off-white solid. TLC system: 10% EtOAc in hexane Rf : 0.8 LCMS (ESI): m/z 369 [M+H]+ |
| 42.1% |
|
Compound 7 (4.40 g, 20.4 mmol) was dissolved in 30 mL of dry DMF,Add sodium hydrogen(1.30 g, 32.6 mol) and stirred for 30 minutes.P-toluenesulfonyl chloride(5.78 g, 30.6 mmol) and reacted for 4 hours.After the reaction, it was poured into an ice-water mixture, a solid precipitated, and filtered.The filter cake was washed with petroleum ether, and the crude product was recrystallized from ethyl acetate to obtain 3.10 g of compound 8.Yield: 42.1%. |
| 8.26 g |
|
Formation of 3-bromo-5-fluoro-l-(p-tolylsulfonyl)pyrrolo[2,3-b]pyridine (22a)3-bromo-5-fluoro-lH-pyrrolo[2,3-b]pyridine (5.0 g, 23.3 mmol) was dissolved in DMF (37.5 mL) and cooled to 0 C. Sodium hydride (1.5 g, 37.2 mmol) was added and the reaction mixture was stirred for 10 minutes and then treated with tosyl chloride (6.6 g, 34.9 mmol). The mixture was stirred for 30 minutes at 0 C and then at room temperature for another 90 minutes. The reaction mixture was poured into water (100 mL) and the resulting solid was collected, washed with water and hexanes three times and dried in vacuo to afford 8.26 g of 3-bromo-5- fiuoro-l-(p-tolylsulfonyl)pyrrolo[2,3-b]pyridine, 22a: 1H NMR (300 MHz, DMSO- |
| 3.1 g |
|
Preparation of compound 3: Compound 2 (4.40 g, 20.4 mmol) was dissolved in 30 mL of dry DMF, sodium hydrogen (1.30 g, 32.6 mol) was added and stirred for 30 min. Then p-toluenesulfonyl chloride (TsCl, 5.78 g, 30.6 mmol) was added and reacted for 4 hours.After the reaction was completed, it was poured into an ice-water mixture, a solid was precipitated, and the filter cake was washed with petroleum ether.The crude product was recrystallized from ethyl acetate to give 3.10 g of Compound 3. Yield: 42.1%. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping