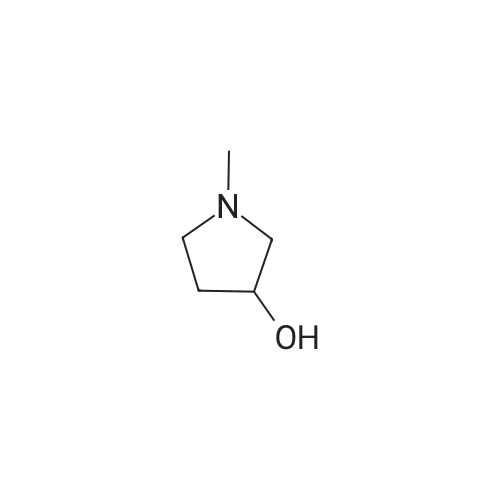
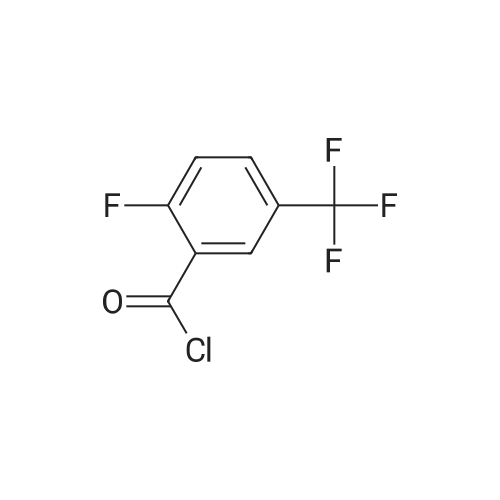
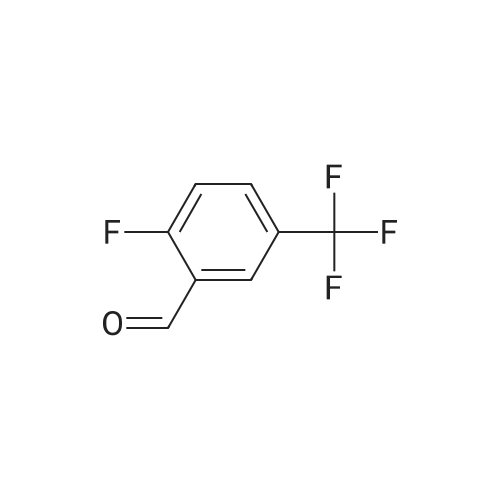
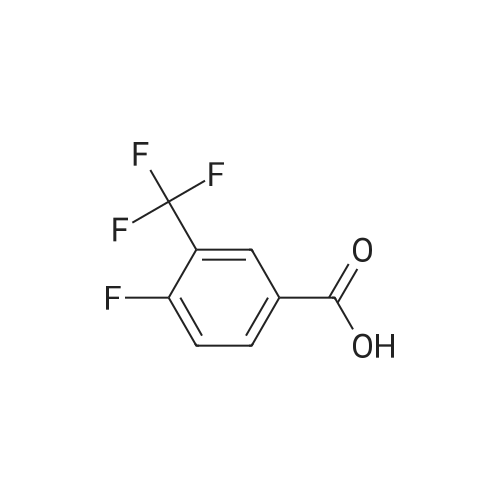
|
With oxalyl dichloride; N,N-dimethyl-formamide; In dichloromethane; at 20℃; for 4h; |
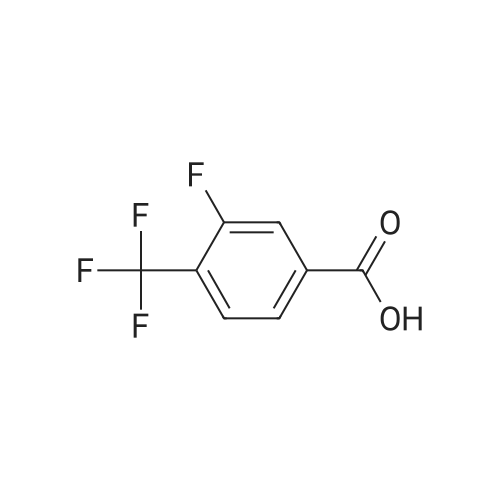
General procedure: To a suspension of the carboxylic acid (5.00 mmol) in CH2Cl2 (15 mL) was added DMF (2 drops) followed by oxalylchloride (0.530 mL, 6.25 mmol). The reaction mixture was stirred at rt for 4 h, and then evaporated to dryness. Theacid chloride thus prepared was dissolved in CH2Cl2 (15 mL) at 0 C. Triethylamine (2.10 mL, 15.0 mmol) and added,followed by either ethylamine hydrochloride, benzylamine, or 2,4-dimethoxybenzylamine (7.50 mmol). The reactionmixture was allowed to warm to rt and stirred for a further 15 h. Water (50 mL) was added, and the aqueous phaseextracted with CH2Cl2 (3 × 50 mL). The combined organics were washed with saturated Na2CO3 (20 mL), dried overMgSO4, subjected to filtration, and concentrated in vacuo. The crude product was purified by flash chromatographyon silica gel, eluting with EtOAc/hexanes/CH2Cl2, to afford the title compound. |
|
With oxalyl dichloride; N,N-dimethyl-formamide; In dichloromethane; at 20℃; for 3h;Inert atmosphere; |
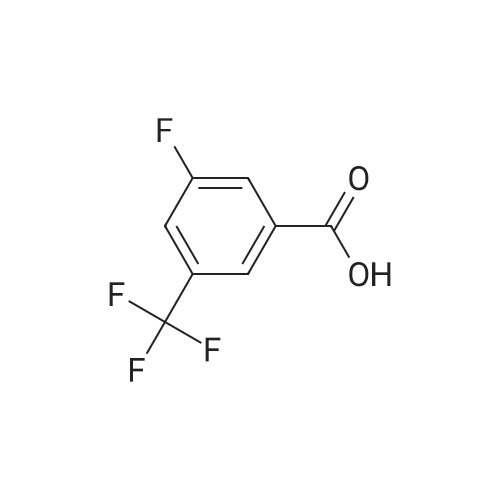
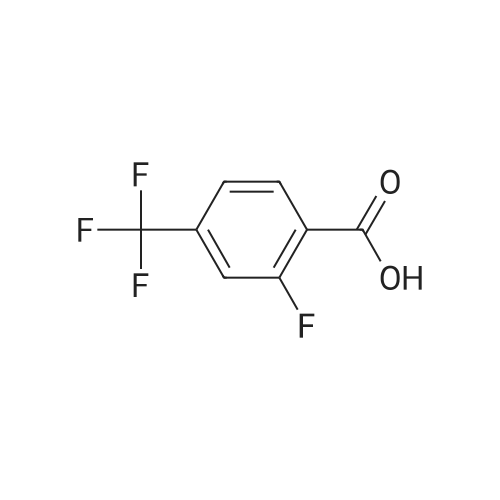
To a solution of 2-fluoro-5-trifluoromethylbenzoic acid (500 mg, 2.4 mmol) in anhydrous dichloromethane (10 mL) was added oxalyl chloride (410 μL, 4.8 mmol) followed by N,N-dimethylformamide (2 drops) and the mixture was stirred at room temperature for 3 hours. The volatiles were removed under a stream of nitrogen and the residue taken up in dichloromethane and added slowly to a solution of 11 (540 mg, 2.4 mmol) and N,N-diisopropylethylamine (1.0 mL, 6.0 mmol) in dichloromethane (10 mL) at 0 C. The mixture was warmed to room temperature and stirred for 4 hours. The mixture was diluted with dichloromethane (30 mL) and washed with saturated aqueous sodium hydrogen carbonate (20 mL) and saturated aqueous ammonium chloride. The organic phase was dried over anhydrous magnesium sulfate, concentrated under reduced pressure and purified by flash column chromatography using ethyl acetate, hexane (0:1 to 3:7) as an eluent to obtain the title compound (660 mg, 67%) as an off-white waxy solid, Rf: 0.45 (2:3 ethyl acetate, hexane); IR (vmax (neat)): 3294, 2962, 1680, 1547, 1326, 1232, 1129, 1092 cm-1; 1H NMR (400 MHz, CDCl3): δ 1.31 (9H, s), 1.62-1.76 (2H, m), 1.80-1.94 (1H, m), 2.00-2.08 (1H, m), 3.68-3.89 (2H, m), 4.11 (1H, dd, J = 15.1, 5.8 Hz), 4.18-4.29 (1H, m), 4.41 (1H, dd, J = 15.4, 1.9 Hz), 6.55 (1H, s), 7.28-7.36 (1H, m), 7.74-7.84 (1H, m), 8.39 (1H, dd, J = 6.9, 2.5 Hz), 10.08 (1H, d, J = 8.1 Hz) ppm; 13C NMR (100 MHz, CDCl3): δ 25.9, 28.1, 30.6, 32.4, 53.0, 68.8, 79.7, 95.6, 117.3 (d, J = 25.3 Hz), 122.4 (d, J = 13.8 Hz), 122.9 (q, J = 272.1 Hz), 128.0 (q, J = 30.3 Hz), 130.1-130.2 (m), 130.7-140.0 (m), 136.4, 159.0 (d, J = 2.8 Hz), 161.0, 161.9 (d, J = 255.2 Hz) ppm; 19F NMR (282 MHz, DMSO-d6): δ -62.3 (CF3), -108.4 (CF) ppm; LRMS (+ESI) m/z: 436.2 ([M+Na]+ 100%), 849.2 ([2M+Na]+ 43%). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping