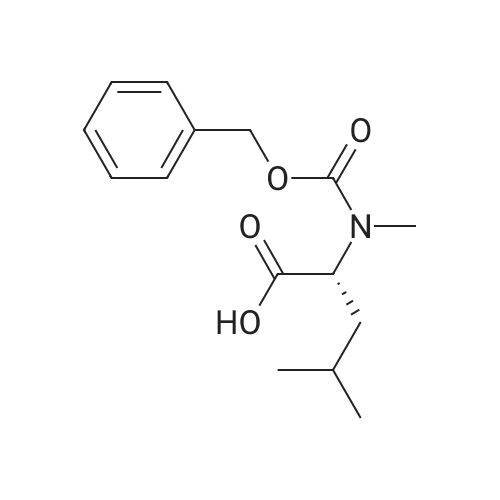
| 74% |
With sodium tetrahydroborate; iodine; In tetrahydrofuran; at 0℃; for 18h;Reflux; |
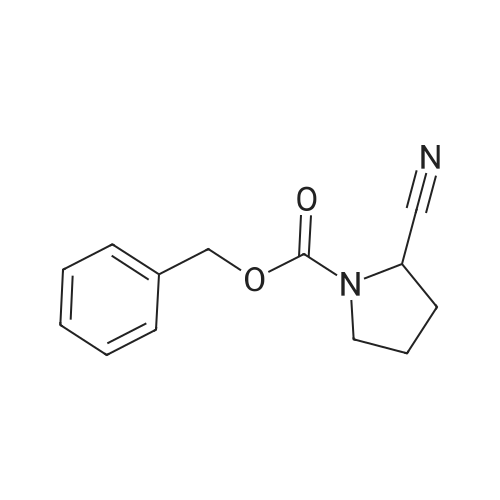
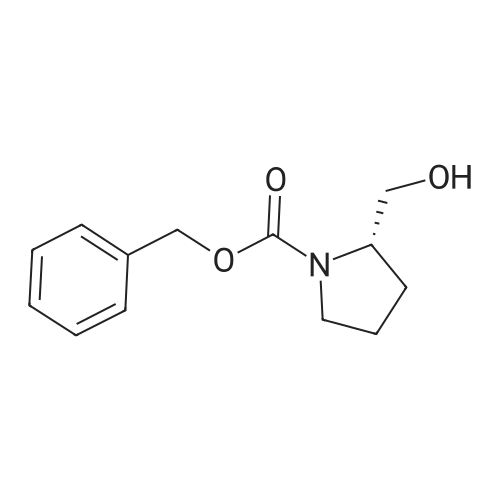
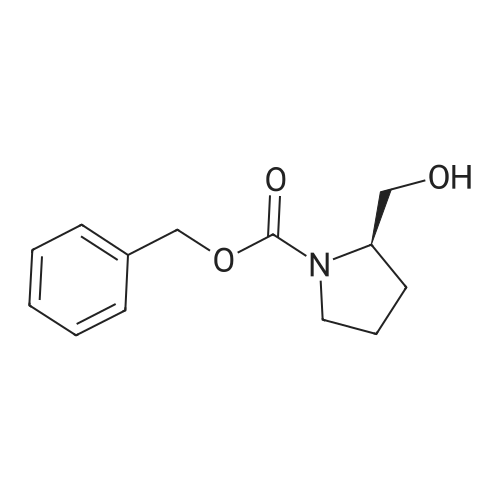
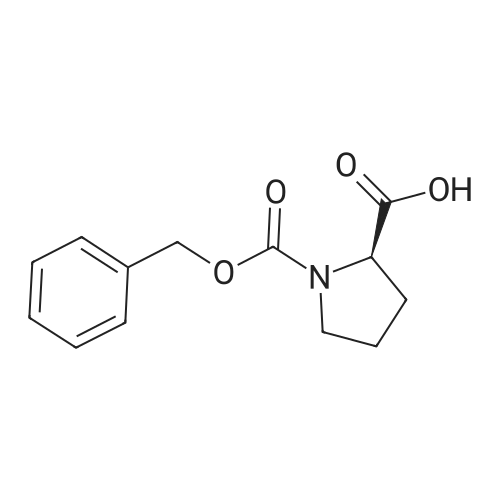
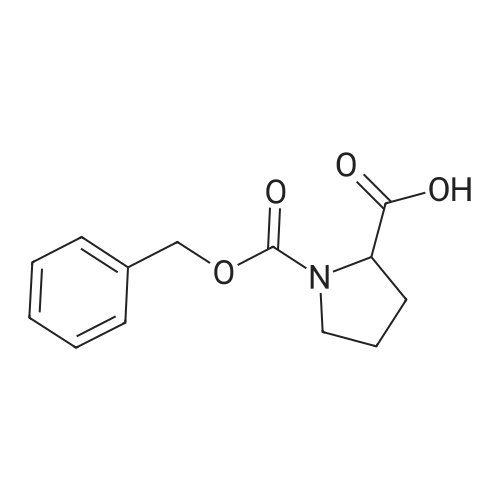
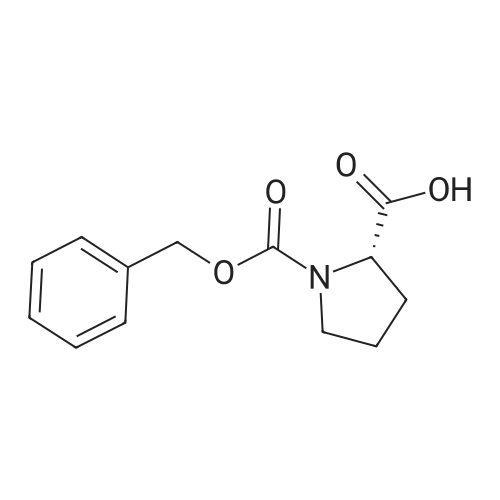
To a solution of Cbz-(S)-proline 7 (10.5 g, 46.25 mmol) in dry THF (100 mL) in a two necked round bottom flask was added NaBH4 (3.98 g, 105.3 mmol) in three portions at 0 C. After stirring the reaction mixture at 0 C for ? hour, I2 (10.70 g, 42.16 mmol) dissolved in dry THF (25 mL) was added drop wise over a period of 30 min. This solution was refluxed for a period of 18 h till the TLC showed complete disappearance of the starting material. The milky reaction mixture was stirred further for 30 min at room temperature followed by the addition of methanol (50 mL) till a clear solution appears. A white paste was obtained after evaporation under reduced pressure. It was further quenched with 1N HCl and extracted with CH2Cl2 (3 × 20 mL). Column chromatography afforded a colorless gummy liquid Cbz-(S)-prolinol 8 (7.55 g, 74% yield). [alpha]D -41.6 (c 1.73, CHCl3) (lit. -41.8 (c 1.4, CHCl3) [22]); IR (Neat): v 3430, 3033, 2953, 2880, 1680, 1418, 1357, 1192, 1105, 1049, 747, 698 cm-1; 1H NMR (200 MHz, CDCl3): delta 1.60-1.72 (m, 1H), 1.77-2.10 (m, 3H), 3.36-3.45 (m, 1H), 3.50-3.66 (m, 3H), 3.91-4.01 (m, 1H), 4.15-4.27 (br s, 1H), 5.11-5.15 (d, 2H, J = 3.77 Hz), 7.25-7.37 (m, 5H); MS (ESI): m/z (%) = 236 (M + H, 100). |
|
With acetic acid; In tetrahydrofuran; methanol; dimethylsulfide; |
Preparation 4 (2S)-1-(Benzyloxycarbonyl)-2-hydroxymethyl pyrolidine STR39 To a stirred solution of 1(benzyloxycarbonyl)-L-proline (15 g) in tetrahydrofuran (150 ml) at 0 C., borane-methylsulfide complex (9 ml, 95% in dimethyl sulfide) was added and the reaction mixture was stirred at room temperature for 8 h. At the end of this time the reaction was quenched with 10% AcOH in methanol and the solvent was removed under reduced pressure. The crude product thus obtained was diluted with CHCl3 and washed with water, dried (CaCl2) and the solvent was removed under reduced pressure to get 11.5 g (81%) of the title compound as a thick liquid. 1 H NMR (CDCl3, 200 MHz): d 1.5-2.2 (m, 4H), 3.3-3.8 (m, 4H), 4.0 (m, 1H), 5.15 (s, 2H), 7.36 (bs, 5H). |
|
In tetrahydrofuran; |
23a. CBZ-L-prolinol To Carbobenzyloxy-L-proline (Aldrich, 9.80 g, 39.3 mmol) in THF (100 mL) at 0 C. was added BH3 -THF complex (Aldrich, 1M in THF, 100 mL, 100 mmol). The mixture was stirred at 0 C. for 2 hours, then at ambient temperature for 16 hr. The reaction mixture was poured into pH 7 phosphate buffer solution (500 mL) and ether (600 mL) and mixed well. The layers were separated and the aqueous portion was extracted with ether (500 mL). The combined organic layers were washed with brine (500 mL), dried (Na2 SO4) and filtered. The solvents were evaporated in vacuo leaving the crude carbobenzyloxy-L-prolinol as an oil (10.5 g). |
|
In tetrahydrofuran; |
23a. CBZ-L-prolinol To Carbobenzyloxy-L-proline (Aldrich, 9.80 g, 39.3 mmol)in THF (100 mL) at 0 C. was added BH3 -THF complex (Aldrich, 1M in THF, 100 mL, 100 mmol). The mixture was stirred at 0 C. for 2 hours, then at ambient temperature for 16 hr. The reaction mixture was poured into pH 7 phosphate buffer solution (500 mL) and ether (600 mL) and mixed well. The layers were separated and the aqueous portion was extracted with ether (500 mL). The combined organic layers were washed with brine (500 mL),dried (Na2 SO4) and filtered. The solvents were evaporated in vacuo leaving the crude carbobenzyloxy-L-prolinol as an oil (10.5 g). |
|
In tetrahydrofuran; |
23a. CBZ-L-prolinol To Carbobenzyloxy-L-proline (Aldrich, 9.80 g, 39.3 mmol)in THF (100 mL) at 0 C. was added BH3 -THF complex (Aldrich, 1M in THF, 100 mL, 100 mmol). The mixture was stirred at 0 C. for 2 hours, then at ambient temperature for 16 hr. The reaction mixture was poured into pH 7 phosphate buffer solution (500 mL) and ether (600 mL) and mixed well. The layers were separated and the aqueous portion was extracted with ether (500 mL). The combined organic layers were washed with brine (500 mL),dried (Na2 SO4) and filtered. The solvents were evaporated in vacuo leaving the crude carbobenzyloxy-L-prolinol as an oil (10.5g). |
|
|
Reference Example 55 ; L(-)-proline (17.0 g) was dissolved in tetrahydrofuran (350 ml) and a 1 N aqueous sodium hydroxide solution (296 ml), and then benzyl chlorocarbonate (23.2 ml) was added dropwise thereto at 0C under a nitrogen atmosphere. The resulting mixture was returned to room temperature and stirred overnight. 1 N Hydrochloric acid (296 ml) was added thereto at 0C, and the mixture was extracted with ethyl acetate. The organic layer was washed with saturated brine, and dried over magnesium sulfate. The solvent was distilled off under reduced pressure to give 1-[(benzyloxy)carbonyl]-L-proline, which was as such dissolved in tetrahydrofuran (250 ml), and then triethylamine (26.8 ml) was added thereto. To the resulting solution was added dropwise ethyl chlorocarbonate (16.9 ml) at 0C under a nitrogen atmosphere. The mixture was returned to room temperature and stirred for 1 hour, and the insolubles were removed by filtration. To the filtrate was added dropwise an aqueous sodium borohydride (11.2 g) solution (100 ml) at 0C, and the mixture was returned to room temperature and stirred for 3 hours under a nitrogen atmosphere. Next, water was added thereto, and the mixture was extracted with ethyl acetate twice. The organic layer was washed with saturated brine. The solvent was distilled off under reduced pressure, and the resulting residue was purified by silica gel column chromatography (hexane : ethyl acetate 2 : 1 ? 1 : 4) to give benzyl (2S)-2-(hydroxymethyl)pyrrolidine-1-carboxylate (25.0 g) as a colorless oily material. To a solution of oxalyl chloride (6.23 ml) in dichloromethane (70 ml) was added dropwise a solution of dimethyl sulfoxide (10.9 ml) in dichloromethane (100 ml) at -78C under an argon atmosphere. The mixture was stirred as such for 15 minutes, and then a solution of benzyl (2S)-2-(hydroxymethyl)pyrrolidine-1-carboxylate (12.0 g) in dichloromethane (80 ml) was added dropwise thereto. The mixture was stirred as such for 15 minutes, and then triethylamine (42.7 ml) was added dropwise thereto, followed by stirring as such for 20 minutes. The resulting mixture was returned to room temperature and then water was added thereto, followed by separation. The organic layer was washed with 1 N hydrochloric acid twice, an aqueous 4% sodium carbonate solution and saturated brine, respectively, and then dried over magnesium sulfate. The solvent was distilled off under reduced pressure, and the resulting residue was purified by silica gel column chromatography (hexane : ethyl acetate = 2 : 1 ? 1 : 1) to give benzyl (2S)-2-formylpyrrolidine-1-carboxylate. To [2-(1,3-dioxolan-2-yl)ethyl]triphenylphosphonium bromide (90.4 g) was added tetrahydrofuran (400 ml), further added t-butoxypotassium, and the mixture was stirred at room temperature for 1.5 hours under a nitrogen atmosphere. To the resulting mixture was added dropwise a solution of benzyl (2S)-2-formylpyrrolidine-1-carboxylate in tetrahydrofuran (200 ml), and the mixture was stirred at room temperature for 1 hour and at 50C for 3 hours under a nitrogen atmosphere. After returning to room temperature, water was added thereto and the mixture was extracted with ethyl acetate. The organic layer was washed with saturated brine, and dried over magnesium sulfate. The solvent was distilled off under reduced pressure, and the resulting residue was purified by silica gel column chromatography (hexane : ethyl acetate = 3 : 1 ? 2 : 1) to give benzyl (2S)-2-[3-(1,3-dioxolan-2-yl)-1-propenyl]pyrrolidine-1-carboxylate (15.1 g). Benzyl (2S)-2-[3-(1,3-dioxolan-2-yl)-1-propenyl]pyrrolidine-1-carboxylate (15.0 g) was dissolved in ethanol (200 ml), and then 10% palladium carbon (4.0 g, water content: 50%) was added thereto, followed by overnight stirring at room temperature under a hydrogen atmosphere. The insolubles were removed by filtration, and then the solvent was distilled off under reduced pressure. The resulting residue was dissolved in tetrahydrofuran (200 ml) and a 1 N aqueous sodium hydroxide solution (150 ml), and then benzyl chlorocarbonate (10.1 ml) was added dropwise thereto at 0C under a nitrogen atmosphere. The mixture was returned to room temperature, stirred for 3 hours and extracted with ethyl acetate. The organic layer was washed with saturated brine, and dried over magnesium sulfate. The solvent was distilled off under reduced pressure, and the resulting residue was purified by silica gel column chromatography (hexane : ethyl acetate = 7 : 1) to give benzyl (2R)-2-[3-(1,3-dioxolan-2-yl)propyl]pyrrolidine-1-carboxylate (13.6 g). Benzyl (2R)-2-[3-(1,3-dioxolan-2-yl)propyl]pyrrolidine-1-carboxylate (13.0 g) was dissolved in tetrahydrofuran (130 ml), and then 2 N hydrochloric acid (305 ml) was added thereto at 0C. After stirring the resulting mixture at room temperature for 3.5 hours, a 1 N aqueous sodium hydroxide solution (610 ml) was added thereto at 0C. The mixture was extracted with ethyl acetate, the organic layer was then washed with satur... |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping