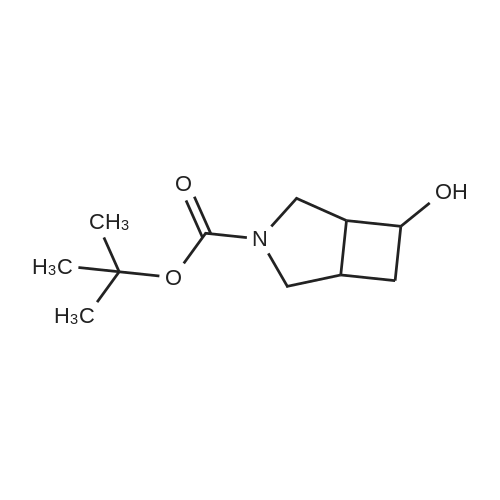
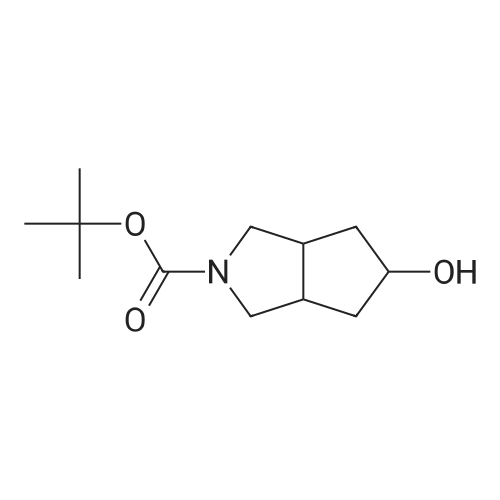
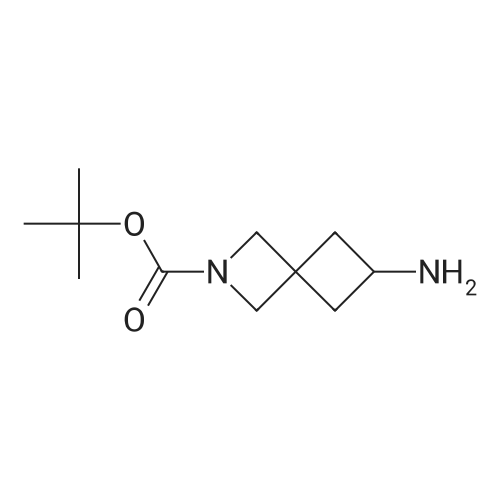
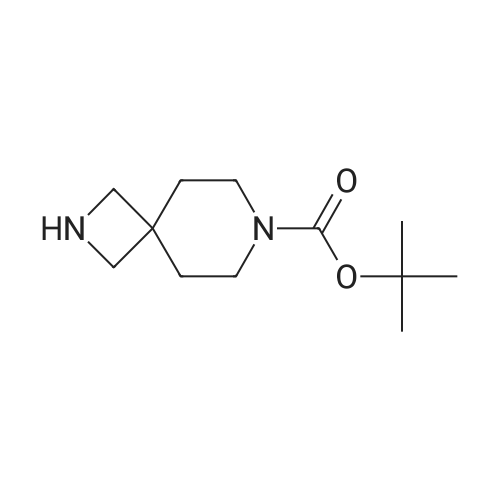
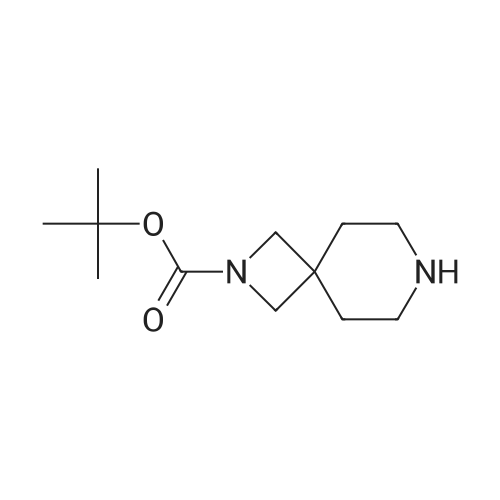
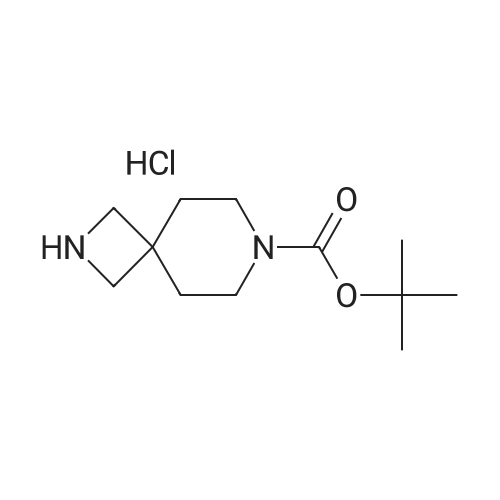
| 93.7% |
With methanol; sodium tetrahydroborate; at 0 - 25℃; for 1h;Inert atmosphere; |
Tert-butyl 6-oxo-2-azaspiro[3.3]heptane-2-carboxylate (4.22 g, 20 mmol) was added in methanol (30 mL), cooled under nitrogen gas protection to 0C, and sodium borohydride (1.52 g, 40 mmol) was added. After addition, the reaction solution was heated to 25C and stirred for 1 h, after completing reaction as measured by LC-MS, water (1 mL) was added to quench reaction, solvent was removed by vacuum distillation, water (100 mL) and ethyl acetate (100 mL) were added, the phases were separated, the organic phase was washed with hydrochloric acid (1 mol/L, 50 mL), dried over anhydrous sodium sulfate, filtered, the filtrate was concentrated to obtain the title compound in white color (4.0 g, yield 93.7 %). |
| 89.2% |
With sodium tetrahydroborate; In ethyl acetate; at 0 - 20℃; for 0.666667h; |
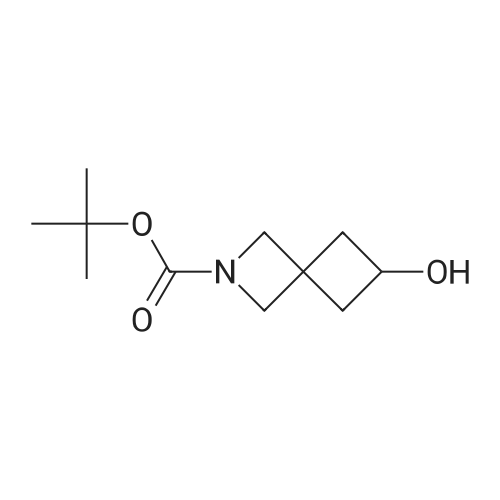
t-Butyl 2-oxo-6-azaspiro[3.3]heptane-6-carboxylate (2.00 g, 9.47 mmol) was dissolvedin EtOAc (40 mL). The mixture was cooled to 0 C, and sodium borohydride (548 mg, 14.19 mmol) was added in portions, the mixture was stirred for 10 min at 0 C, and then further stirredat rt for 30 min. The mixture was cooled to 0 C, and quenched with saturated aqueousammonium chloride (2.0 mL). The mixture was stirred for 30 min, water (20 mL) and ethylacetate (50 mL) were added, and the mixture was stirred for 10 min. After the mixture waspartitioned, the water phase was extracted with ethyl acetate (100 mL x 2). The combined organicphases were washed with saturated aqueous NaCl and dried over anhydrous sodium sulfate, andfiltered. The filtrate was concentrated in vacuo to get the title compound as an off-white solid(1.80 g, 89.2%).MS (ESI, pos. ion) m/z:236.1 [M+Ht |
|
With methanol; sodium tetrahydroborate; at 0 - 20℃; for 1h; |
0.494 g (13.06 mmoles) of sodium borohydride is added in portions at 0 C. to a solution of 2.30 g (10.89 mmoles) of tert-butyl 6-oxo-2-aza-spiro[3.3]-heptane-2-carboxylate, obtained in stage 10.2, in 55 ml of methanol. The reaction mixture is stirred for 1 hr at ambient temperature. After evaporation of the solvent, water is added to the reaction mixture, the aqueous phase is separated, it is extracted several times with dichloromethane, and the combined organic phases are washed with a saturated aqueous solution of sodium chloride, dried over sodium sulphate and the filtrate is concentrated under reduced pressure. After crystallisation of the residue in diisopropyl ether, filtration of the solid obtained and drying under vacuum at 60 C., 2.24 g of product are obtained in the form of a beige powder.1H NMR (DMSO) delta (ppm): 5.00 (d, 1H); 3.95 (hex, 1H); 3.75 (d, 4H); 2.40 (m, 2H); 1.95 (m, 2H); 1.40 (s, 9H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping