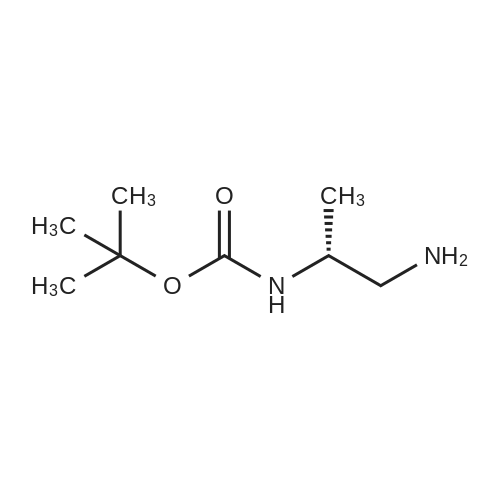
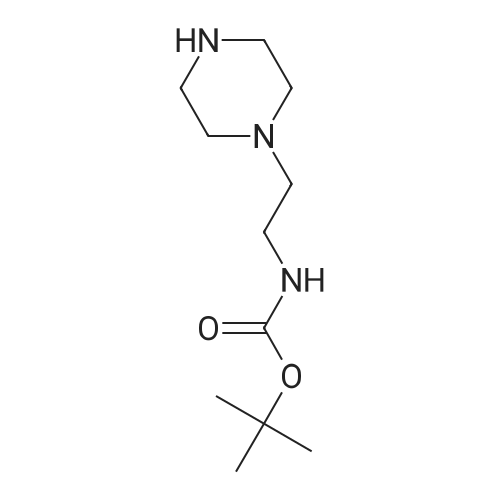
Alternatived Products of [ 113283-93-5 ]
Product Details of [ 113283-93-5 ]
| CAS No. : | 113283-93-5 |
MDL No. : | MFCD08729301 |
| Formula : |
C9H20N2O2
|
Boiling Point : |
No data available |
| Linear Structure Formula : | - |
InChI Key : | JMIGZQVLLZKDIS-UHFFFAOYSA-N |
| M.W : |
188.27
|
Pubchem ID : | 13855174 |
| Synonyms : |
|
Safety of [ 113283-93-5 ]
Application In Synthesis of [ 113283-93-5 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 113283-93-5 ]
- 1
-
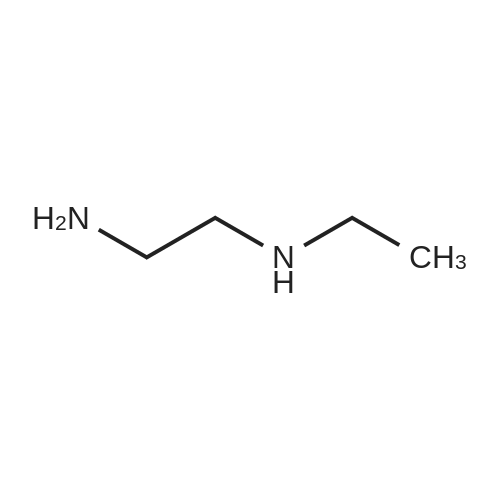 [ 110-72-5 ]
[ 110-72-5 ]

-
 [ 24424-99-5 ]
[ 24424-99-5 ]

-
 [ 113283-93-5 ]
[ 113283-93-5 ]
| Yield | Reaction Conditions | Operation in experiment |
|
|
Reference Example 15 N-(2-aminoethyl)-N-ethyl-2,2,2-trifluoroacetamide hydrochloride According to the method described in Synthetic Communications, 23(17), 2443-2449 (1993), tert-butyl N-[2-(ethylamino)ethyl]carbamate (3.06 g, 16.25 mmol) was obtained from N-ethylethylenediamine (8.36 g) and di-tert-butyl dicarbonate (6.25 g). |
| 3.2 g |
In tetrahydrofuran; at 0 - 20℃; |
In a round bottom flask, N-ethylethylenediamine (8.82 g) was dissolved in 200 ml of anhydrous THF, the solution was cooled to 0-5 C. and di-tert-butyl dicarbonate (6.54 g) dissolved in 60 ml of THF was added during one hour. The mixture was stirred overnight at room temperature. Next, the solvent was distilled off, the residue was dissolved in saturated NaCl solution (60 ml) and the product was extracted with dichloromethane (3*60 ml). The extract was washed with a saturated NaCl solution (60 ml) and dried with anhydrous MgSO4. Next, the solvent was distilled off under reduced pressure and the obtained product was recrystallized from hexane giving 3.2 g of the desired product. |
Reference:
[1]Synthetic Communications,2007,vol. 37,p. 737 - 742
[2]Synthetic Communications,1993,vol. 23,p. 2443 - 2449
[3]Patent: US2012/196824,2012,A1
[4]Patent: US10220020,2019,B2 .Location in patent: Page/Page column 63-64
- 2
-
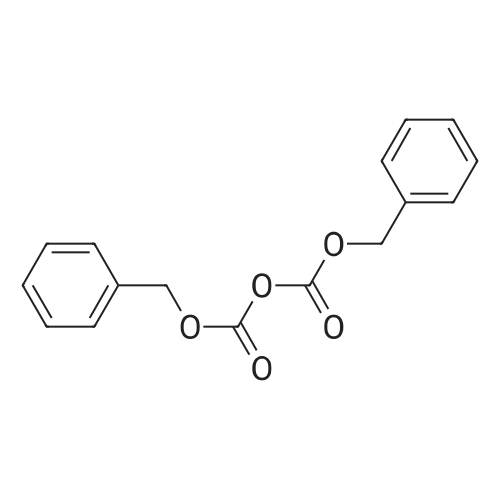 [ 31139-36-3 ]
[ 31139-36-3 ]

-
 [ 113283-93-5 ]
[ 113283-93-5 ]

-
 [ 113283-95-7 ]
[ 113283-95-7 ]
- 3
-
 [ 113283-91-3 ]
[ 113283-91-3 ]

-
 [ 113283-93-5 ]
[ 113283-93-5 ]
- 4
-
 [ 98-88-4 ]
[ 98-88-4 ]

-
 [ 113283-93-5 ]
[ 113283-93-5 ]

-
[2-(Benzoyl-ethyl-amino)-ethyl]-carbamic acid tert-butyl ester
[ No CAS ]
- 5
-
 [ 113283-93-5 ]
[ 113283-93-5 ]

-
 [ 98-59-9 ]
[ 98-59-9 ]

-
 [ 135250-93-0 ]
[ 135250-93-0 ]
- 6
-
 [ 110-72-5 ]
[ 110-72-5 ]

-
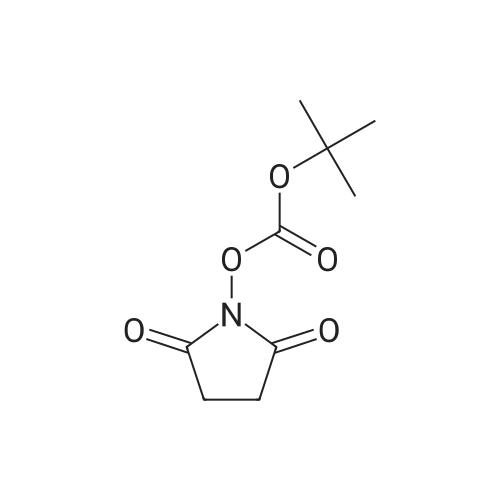 [ 13139-12-3 ]
[ 13139-12-3 ]

-
 [ 113283-93-5 ]
[ 113283-93-5 ]
- 7
-
 [ 113283-93-5 ]
[ 113283-93-5 ]

-
3-iodo-6-(2-methoxyphenyl)-5-methylpyridazine
[ No CAS ]

-
N-1-ethyl-N-1-[6-(2-methoxy-phenyl)-5-methyl-pyridazin-3-yl]-ethane-1,2-diamine
[ No CAS ]
- 8
-
 [ 113283-93-5 ]
[ 113283-93-5 ]

-
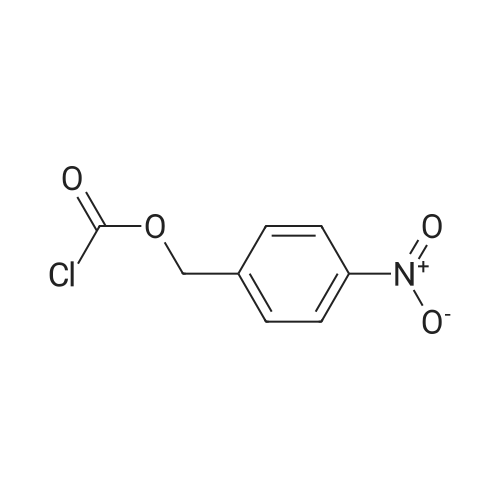 [ 4457-32-3 ]
[ 4457-32-3 ]

-
N-(tert-butoxycarbonyl)-2-[ethyl(4-nitrobenzyloxycarbonyl)amino]ethylamine
[ No CAS ]
- 9
-
 [ 24424-99-5 ]
[ 24424-99-5 ]

-
 [ 113283-93-5 ]
[ 113283-93-5 ]
- 10
-
 [ 110-72-5 ]
[ 110-72-5 ]

-
CaH
[ No CAS ]

-
 [ 24424-99-5 ]
[ 24424-99-5 ]

-
 [ 113283-93-5 ]
[ 113283-93-5 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With sodium chloride; In tetrahydrofuran; |
Step 1. Synthesis of (2-ethylaminoethylearbamic acid, 1,1-dimethylethyl ester) (3) Refer to . A 25 g (0.28 mol) quantity of N-ethylethylenediamine (1) was placed in a dried 500 ml rb flask. THF, 250 ml, previously dried by distillation from CaH, was added via cannula. The flask was kept under argon and cooled in an ice bath for 20 minutes. A dried addition funnel was charged with 50 ml THF to which was added 29.3 ml (0.13 mol) of di-tert-butyl dicarbonate (2). This mixture was added slowly dropwise to the stirred amine. After addition was complete, the reaction was removed from the ice bath and allowed to stir and reach ambient temperature overnight. The volatiles were removed by rotary evaporation. Saturated NaCl (50 ml) was added, and the result extracted with 4*100 ml ethyl acetate. The combined organic fractions were dried over Na2SO4 overnight. The drying agent was removed by filtration and the volatiles removed by rotary evaporation to yield 2-ethylaminoethylcarbamic acid, 1,1 -dimethylethyl ester (3; 27.55 g, 0.15 mol). The material was used without further purification. |
- 11
-
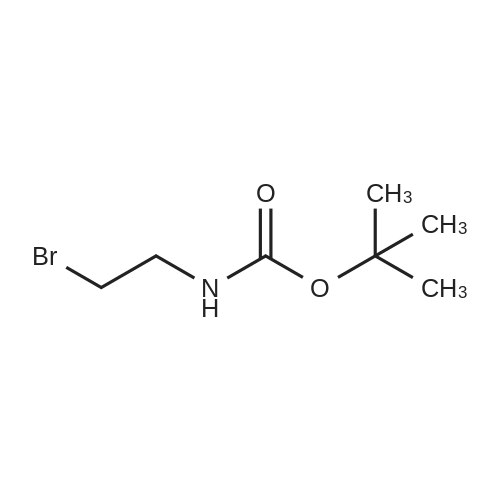 [ 39684-80-5 ]
[ 39684-80-5 ]

-
 [ 75-04-7 ]
[ 75-04-7 ]

-
 [ 113283-93-5 ]
[ 113283-93-5 ]
- 12
-
 [ 1203701-93-2 ]
[ 1203701-93-2 ]

-
 [ 113283-93-5 ]
[ 113283-93-5 ]

-
 [ 1203701-96-5 ]
[ 1203701-96-5 ]
- 13
-
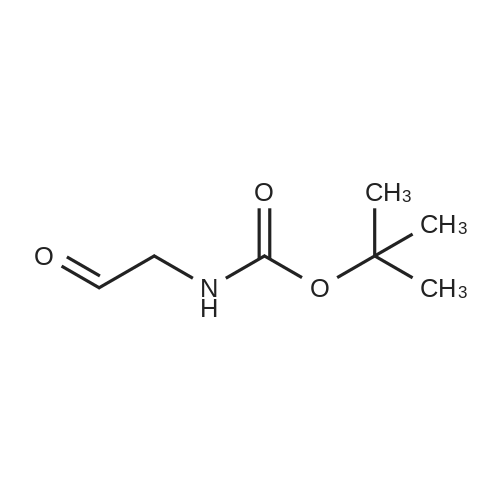 [ 89711-08-0 ]
[ 89711-08-0 ]

-
 [ 75-04-7 ]
[ 75-04-7 ]

-
 [ 113283-93-5 ]
[ 113283-93-5 ]
| Yield | Reaction Conditions | Operation in experiment |
|
|
A solution of the aldehyde A1IJl g, 18.85 mmol) in MeOH (20 ml) was treated with ethylamine (10.37 ml, 20.73 mmol) and stirred at RT for 5 min. The mixture was treated with sodium borohydride (0.856 g, 22.62 mmol). After stirring for 5 min, the mixture was quenched with water and concentrated. The residue was diluted with water, saturated with NaCl, and extracted twice with THF. The organic phase was dried over Na2SO4, filtered and concentrated to yield A-2 as a colorless cloudy oil. |
- 14
-
 [ 1104546-96-4 ]
[ 1104546-96-4 ]

-
 [ 113283-93-5 ]
[ 113283-93-5 ]

-
 [ 1104546-97-5 ]
[ 1104546-97-5 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With triethylamine; In tetrahydrofuran; at 20℃; for 0.75h; |
A solution of the amine A1I (425 mg, 2.256 mmol) and TEA (0.629 ml, 4.51 mmol) in THF (10 ml) was treated slowly with acid chloride (500 mg, 2.256 mmol) and stirred at RT for 45 min. The mixture was diluted with EtOAc and was washed with water and brine. The organic phase was dried over Na2SO4, filtered and concentrated. The crude material was purified by gradient elution on silica gel (0 to 55% EtOAc in Hex) to yield A-4 as a colorless film. Data for AA: LRMS m/z (M+H): 374.01 |
| Yield | Reaction Conditions | Operation in experiment |
| 11% |
In tetrahydrofuran; at 0 - 20℃; for 18h; |
General procedure: tert-Butyl (3-(methylami no)propyl)carbamate A solution of N-methyl-i ,3-propanediamine (6 g, 67.9 mmol) in THF (50 mL) was cooled to 0 C, and a solution of di-tert-butyl dicarbonate (4.6 mL, 20.3 mmol) in THF (50 mL) was added dropwise. The reaction mixture was allowed to come to room temperature, further stirred for 18 h, and concentrated under reduced pressure. Thecrude product was purified by flash column chromatography (silica gel 230-400 mesh, eluent 10% MeOH in CHCI3 to get the undesired regioisomer tert-butyl (3- aminopropyl)(methyl)carbamate, followed by 15% MeOH in CHCI3 to get the desired product) to afford tert-butyl (3-(methylamino)propyl)carbamate (1 g, yield 8%) as a viscous light-yellow liquid. |
- 16
-
(S)-2-[7-(3,5-dimethylphenoxy)-2,5-dioxo-8-(3,4,5-trimethoxybenzoylamino)-2,3,4,5-tetrahydro-1H-benzo[e][1,4]diazepin-3-yl]ethanoic acid
[ No CAS ]

-
 [ 113283-93-5 ]
[ 113283-93-5 ]

-
(S)-N-[3-[2-[(2-aminoethyl)(ethyl)amino]-2-oxoethyl]-7-(3,5-dimethylphenoxy)-2,5-dioxo-2,3,4,5-tetrahydro-1H-benzo[e][1,4]diazepin-8-yl]-3,4,5-trimethoxybenzamide
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
|
The method according to embodiment 53 cases, using the title compound of Example 50 obtained in Preparation 50 (112.7mg, 0.2mmol) and [(ethylamino) methyl] carbamic acidTert-butyl ester (52.8mg, 0.6mmol) reaction, trifluoroacetic acid to remove the Boc protecting derived. Preparation Example 50 The title compound obtained embodiment 50 (112.7mg, 0.2mmol) and DIC (63muL, 0.4mmol), HOSu (46mg, 0.4mmol) were mixed in 2mLTHF reaction at room temperature 12h. Was added diethylamine (62muL, 0.6mmol), the reaction was continued 1h. Column chromatography on silica gel, DCM / MeOH as eluent to give a pale yellow solid 98.7 mg, 79.8% yield. |
- 17
-
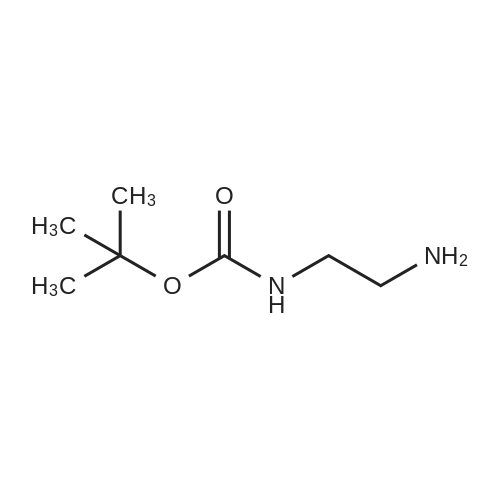 [ 57260-73-8 ]
[ 57260-73-8 ]

-
 [ 75-07-0 ]
[ 75-07-0 ]

-
 [ 113283-93-5 ]
[ 113283-93-5 ]
- 18
-
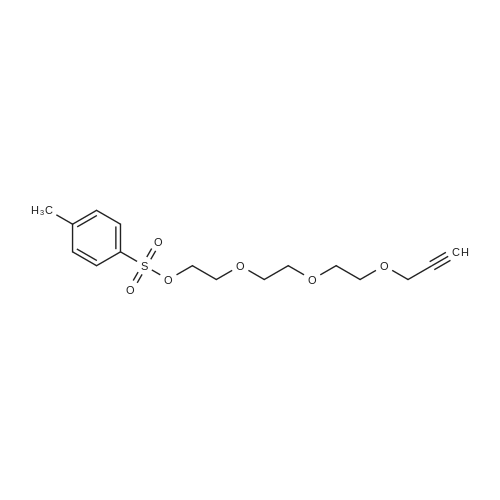 [ 888009-94-7 ]
[ 888009-94-7 ]

-
 [ 113283-93-5 ]
[ 113283-93-5 ]

-
N1-tert-butoxycarbonyl-N2-ethyl-N2-{2-[2-(2-(prop-2-ynyloxy)ethoxy)ethoxy]ethyl}ethane-1,2-diamine
[ No CAS ]
- 19
-
2-(2-{2-[3,5-bis(prop-2-ynyloxy)benzamido]ethoxy}ethoxy)ethyl-4-methylbenzenesulfonate
[ No CAS ]

-
 [ 113283-93-5 ]
[ 113283-93-5 ]

-
N-{2-[2-(2-[2-(tert-butoxycarbonylamino)ethyl](ethyl)amino}ethoxy)ethoxy]ethyl}-3,5-bis(prop-2-ynyloxy)benzamide
[ No CAS ]
- 20
-
C10H7ClO
[ No CAS ]

-
 [ 113283-93-5 ]
[ 113283-93-5 ]

-
tert-butyl (2-(N-ethyl-1-phenylcycloprop-2-ene-1-carboxamido)ethyl)carbamate
[ No CAS ]
- 21
-
C10H6ClFO
[ No CAS ]

-
 [ 113283-93-5 ]
[ 113283-93-5 ]

-
tert-butyl (2-(N-ethyl-1-(4-fluorophenyl)cycloprop-2-ene-1-carboxamido)ethyl)carbamate
[ No CAS ]

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping