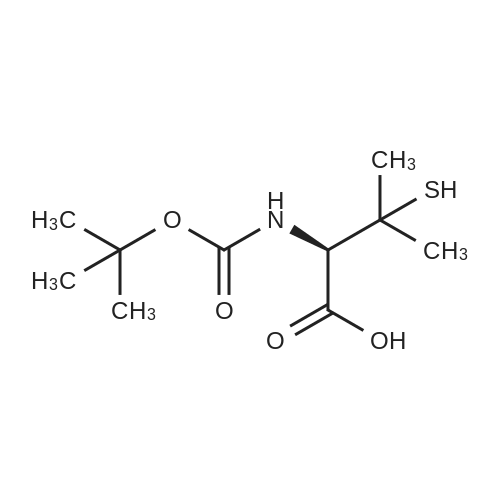
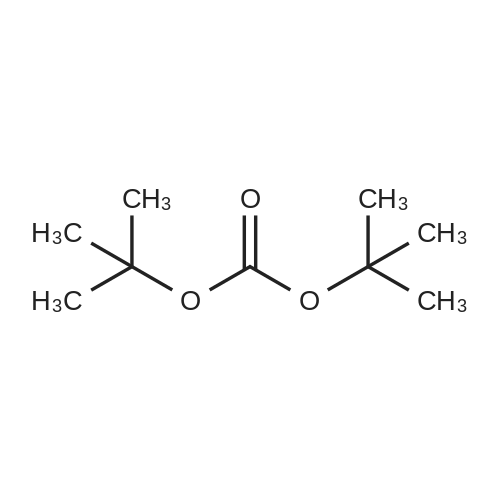
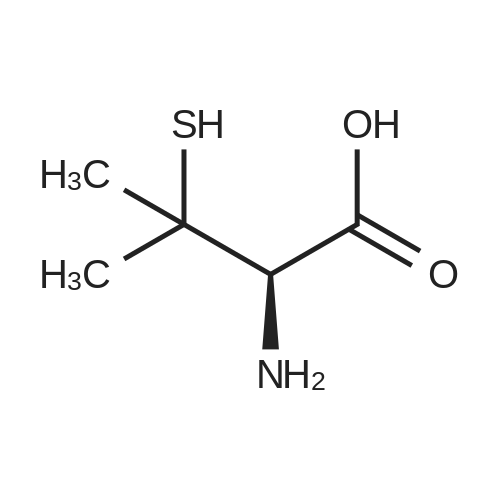
| 88% |
With sodium hydroxide; In 1,4-dioxane; water; at 20℃; for 22h;Cooling with ice; |
To a suspension of (R)-2-amino-3-mercapto-3- methylbutanoic acid (8.0 g, 53.6 mmol) in i,4-dioxane (90 mE) was added a solution of NaOH (4.72 g, 118 mmol) in water (45 mE). The resulting clear solution was cooled with an ice bath and treated with di-t-butyldicarbonate (14.0 g, 64.3 mmol). The reaction mixture was then stirred at rt for20 h. After 2 h, a white precipitate crashed out. The reaction mixture was extracted with ethyl acetate. The aqueous layer was acicified with 2N HC1 solution (pH of 1). The resulting aqueous layer was extracted with ethyl acetate (3x). The organic layer was separated and dried over MgSO4. Thefiltrate was concentrated in vacuo to give a viscous solid. It was vacuum dried on the oil pump to give the title compound as a white solid (11.8 g, 88%). ?H NMR (DMSO-d5) oe 6.90 (d, J=9.2 Hz, iH), 4.07 (d, J=9.2 Hz, iH), 3.57 (s, iH), 3.00 (bt s., iH), 1.40 (s, i5H); MS(ESI) mlz 194.1 (M-56) (M+H). |
|
In sodium carbonate; tert-butyl alcohol; |
N-tert-butoxycarbonyl-L-penicillamine To a solution of L(+)-penicillamine (24 g; 161 mmol) in 10% w/v aqueous sodium carbonate solution (300 ml)was added di-tert-butyl dicarbonate (35.1 g; 161 mmol) in tert-butanol (300 ml). After stirring the reaction mixture for 18 hr at RT, the volume was reduced by approximately one half under reduced pressure and the pH was adjusted to 2 using 1N hydrochloric acid. The resulting slurry was extracted several times with Et2 O, the ethereal layers being combined, dried (MgSO4) and evaporated to give the title compound (36.7 g) as a clear gum. deltaH (CDCl3) 8.65 (1H, br s), 5.50 (1H, d), 4.35 (1H, d), 2.00 (1H, br s), 160 (3H, s), 1.50 (9H, s), and 1.45 (3H, s). |
|
With triethylamine; In N,N-dimethyl-formamide; for 18h; |
To a stirnng suspension of < ?)-2-amino-3-mercapto-3-methylbuianoic add (3.1, 9 g, B0.3 mmo) in D F (20 mL) was added BQC-anhydfide (14.0 mL, 60.3 mmoi) followed by TEA (8.41 mL, 63.3 mrnoi) and the reaction mixture was stirred for 18 hr. Excess solvent was removed under reduced pressure. The. crude product was treated with methyl iodide (18.83 g, 133 mrnoi) in DMF (20 mL) and Cs2C03 (43.2 g. 1 3 mmol) at room temperature for 18 hr. Water (100 mL) was added and the product as extracted with ethyl acetate (2 x 200 mL), dried (NaKCc,) and concentrated under reduced pressure to give compound 3.2 (12.62 g) as a white solid. Subsequent BOG deprotection was achieved by treatment with TFA (20 mL) in CH2CJ2 (20 mL) for 5 hr. The solvent was removed under reduced pressure to give the desired product 3,3 (8.2 g). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping