Probing the Effects of Chirality on Self-Assembling Peptides: Hydrogel Formation, Degradation, Antigen Release, and Adjuvancy
Agrawal, Anushka
;
Euliano, Erin M
;
Pogostin, Brett H
, et al.
Cel. Mol. Bioeng.,2024,17,441-451.
DOI:
10.1007/s12195-024-00806-1
More
Abstract: Introduction Multidomain peptides (MDPs) are amino acid sequences that self-assemble to form supramolecular hydrogels under physiological conditions that have shown promise for a number of biomedical applications. K2(SL)6K2 (“K2”), a widely studied MDP, has demonstrated the ability to enhance the humoral immune response to co-delivered antigen. Herein, we sought to explore the in vitro and in vivo properties of a peptide with the same sequence but opposite chirality (D-K2) since peptides composed of D-amino acids are resistant to protease degradation and potentially more immunostimulatory than their canonical counterparts. Methods K2 and D-K2 hydrogels were characterized and evaluated in vitro using circular dichroism, rheology, cryo-electron microscopy, and fuorescence recovery after photobleaching studies. In vivo experiments in SKH-1 mice were conducted to evaluate both ovalbumin release from the hydrogels and hydrogel degradation. The injection site of the hydrogels was analyzed using histology and humoral immunity was assessed by ELISA. Results In vitro, the enantiomeric hydrogels exhibited similar rheological properties, and fuorescence recovery after pho_x005f_x0002_tobleaching experiments demonstrated that the difusion of ovalbumin (OVA), a model antigen, was similar within both hydrogels. In vivo, K2 and D-K2 peptide hydrogels had similar OVA release rates, both releasing 89% of the antigen within 8 days. Both hydrogels elicited a similar antigen-specifc humoral immune response. However, the in vivo degradation of the D-K2 hydrogel progressed signifcantly slower than K2. After 4 weeks in vivo, only 23±7% of the K2 hydrogel remained at the injection site compared to 94±7% of the D-K2 hydrogel, likely due to their diferent protease susceptibilities. Conclusion Taken together, these data suggest that peptide chirality can be a useful tool for increasing hydrogel residence time for biomedical applications that would beneft from long persistence times and that, if an antigen releases over a suf_x005f_x0002_fciently short period, release can be largely independent of degradation rate, though slower-difusing payloads may exhibit degradation rate dependence.
Keywords:
Hydrogel ;
Chirality ;
Degradation ;
Drug delivery ;
Peptides ;
Adjuvants
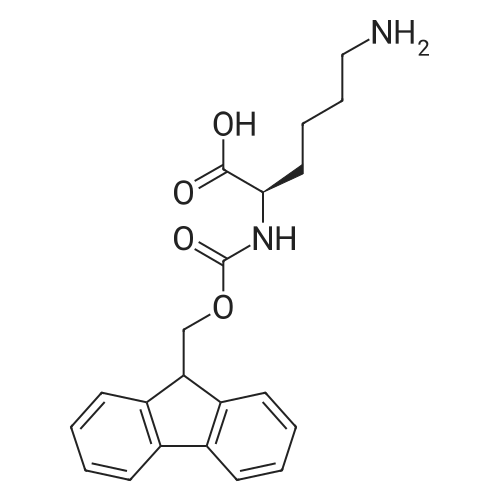
Purchased from AmBeed:
73724-45-5 ;
35661-60-0 ;
114360-54-2 ;
116861-26-8 ;
110990-08-4


 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping