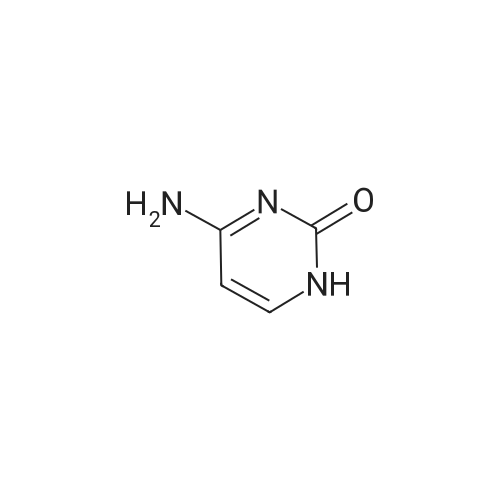
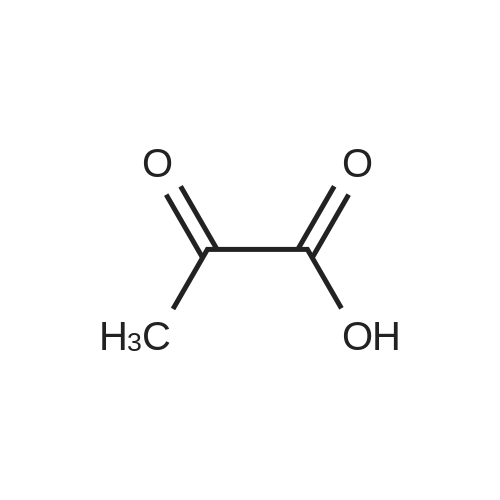
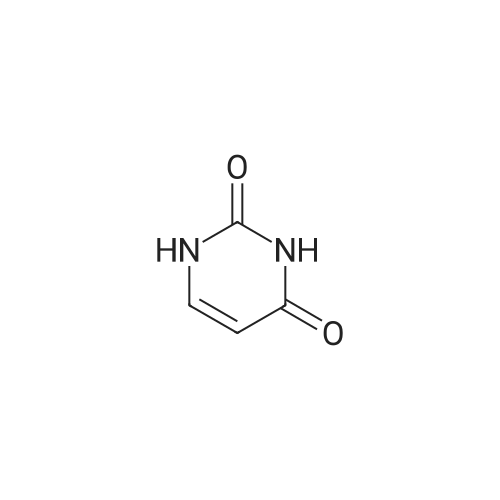
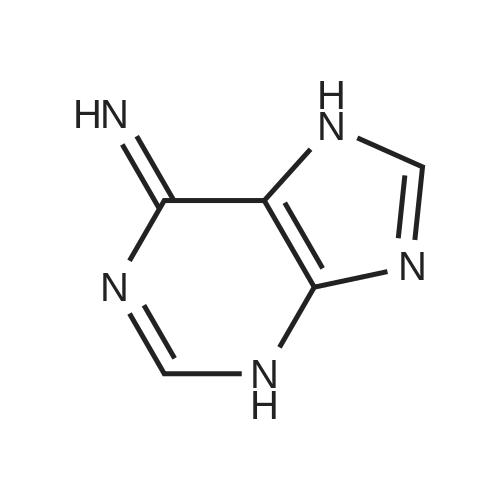
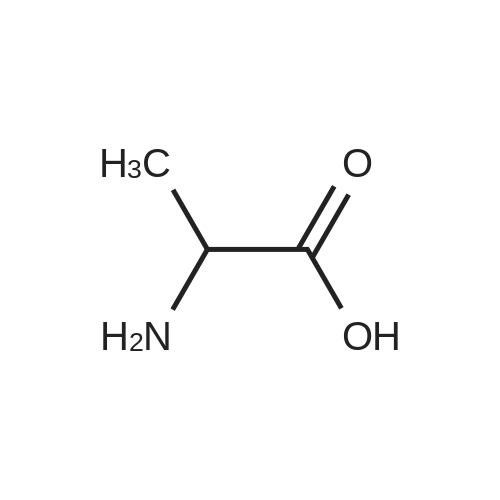
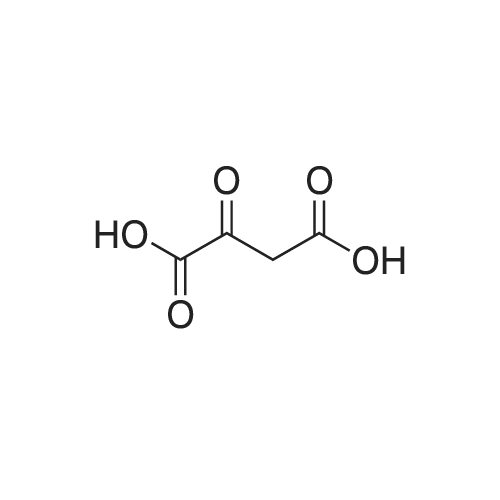
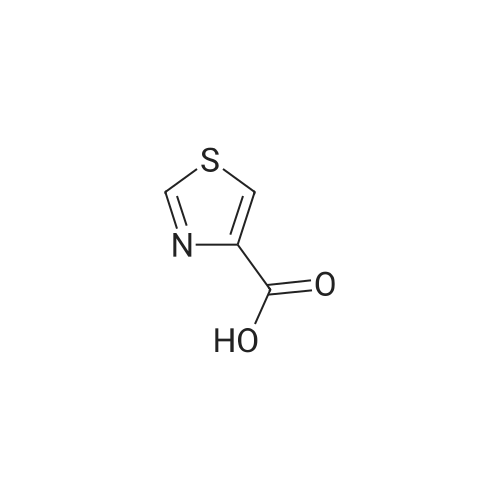
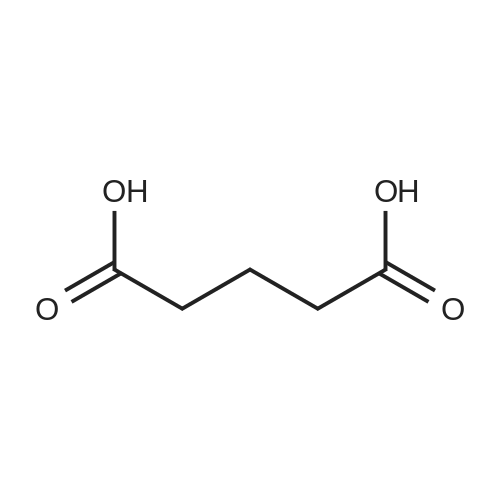
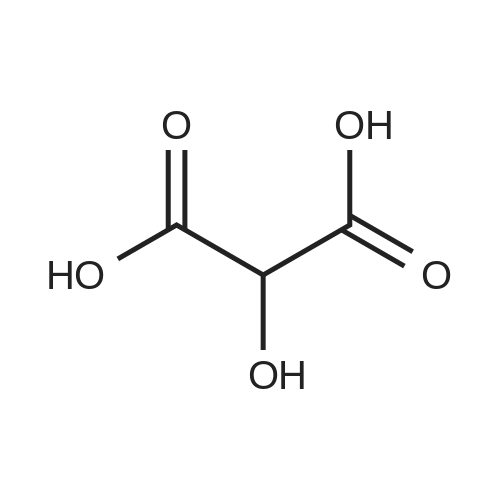
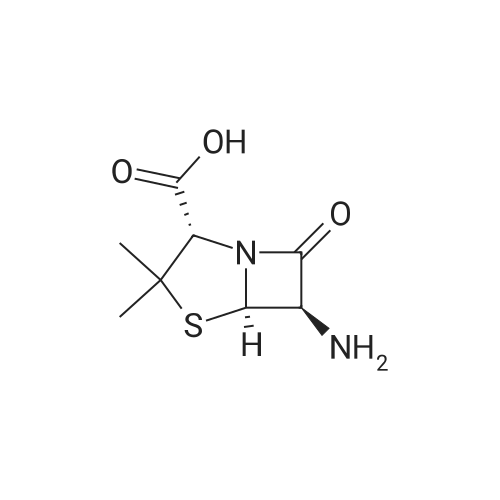
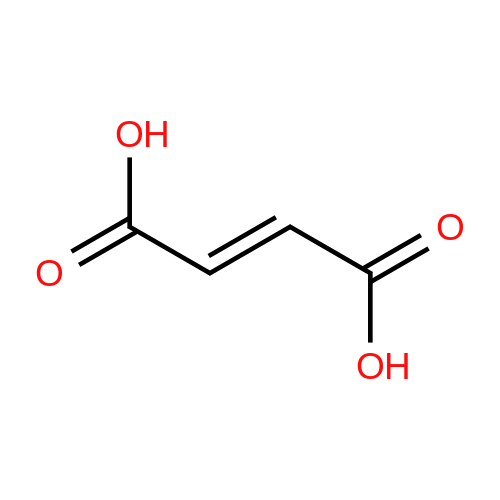
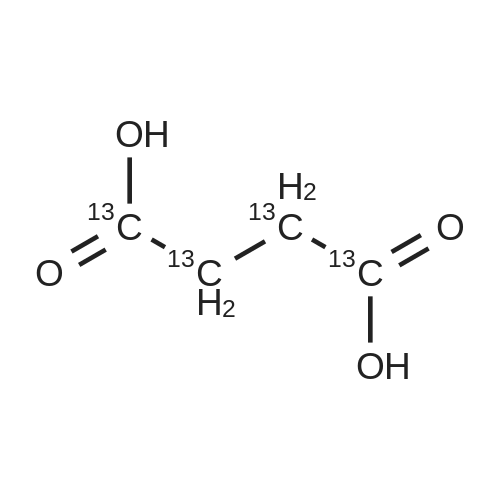
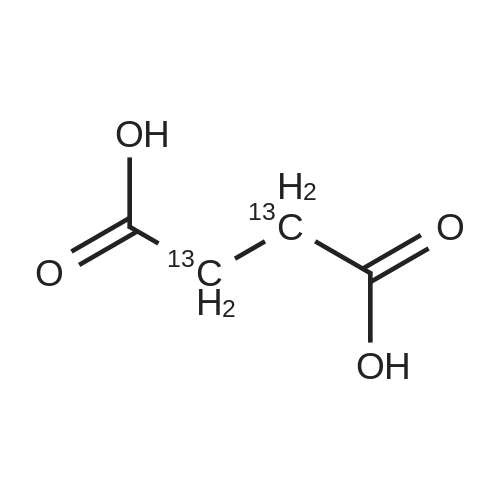
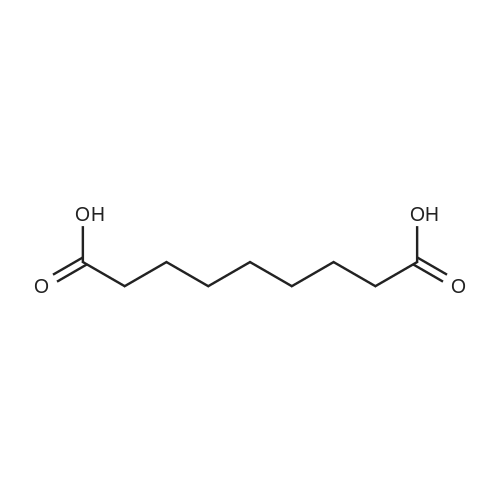
| 85% |
In isopropyl alcohol; at 45 - 50℃; for 1h;Product distribution / selectivity; |
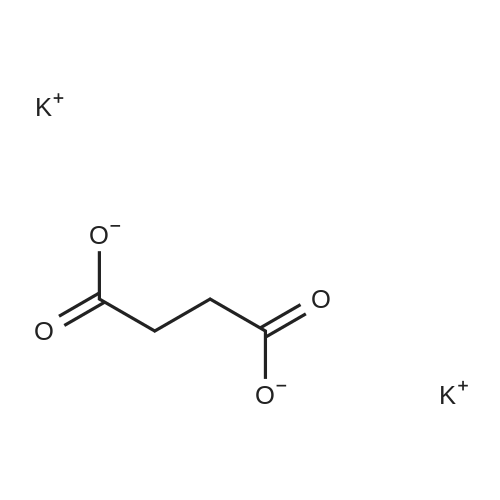
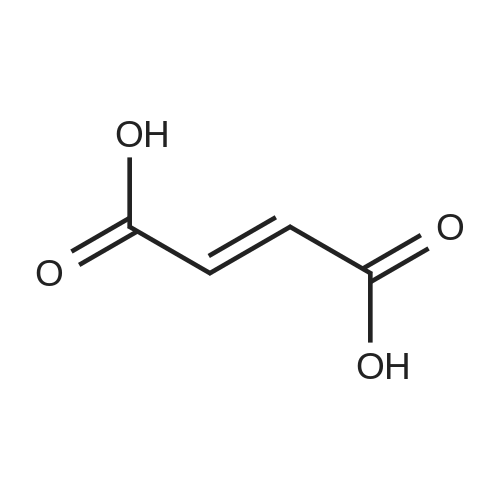
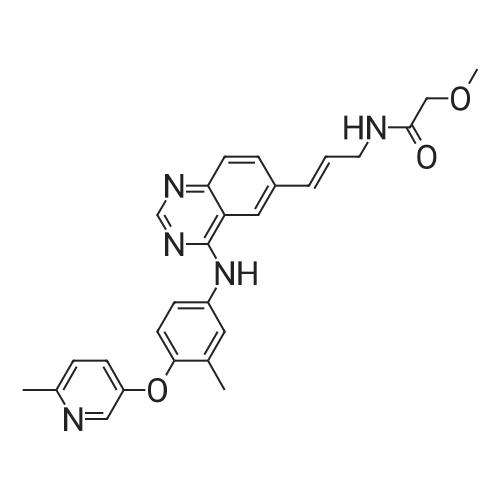
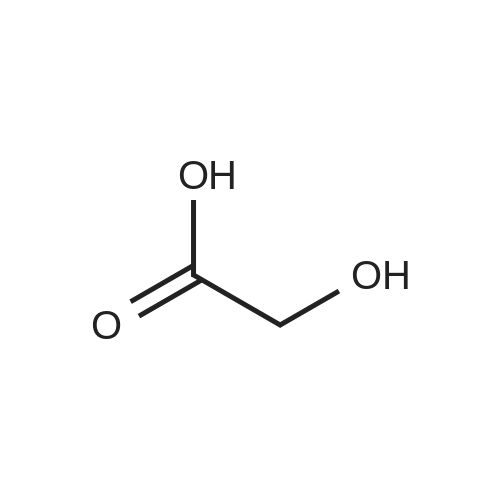
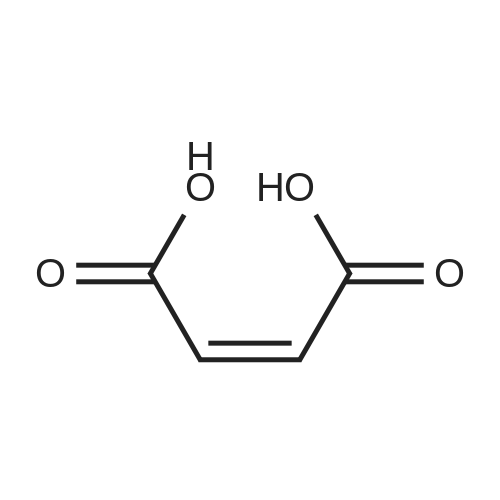
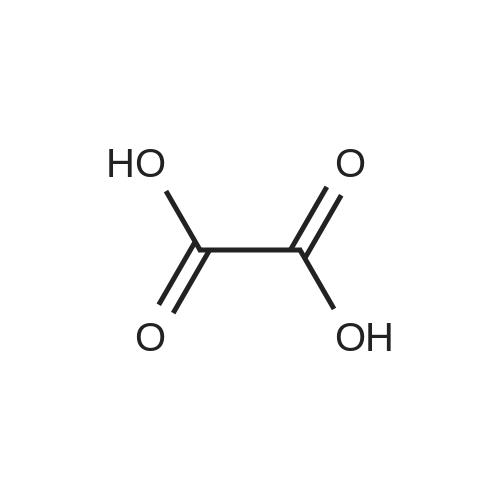
A 50 mL round bottom flask equipped with a condenser, thermometer, and a magnetic spin bar for agitation was charged with <strong>[383432-38-0]E-2-Methoxy-N-(3-{4-[3-methyl-4-(6-methyl-pyridin-3-yloxy)-phenylamino]-quinazolin-6-yl}-allyl)-acetamide</strong> free base (2.0, 1.0 equiv.) and 2-propanol (20 mL). The mixture was heated to solution. A separate 75 mL reactor was charged with succinic acid (0.91 g, 1.8 equivalents) and 2-propanol (10 mL) and warmed to about 45 C. The free base solution was vacuum filtered to remove any solids and was added to the succinic acid solution over about 10 minutes. The resulting slurry was stirred at 45-50 C. for about 1 hour. The slurry was cooled to 20 C. and stirred overnight. The product was isolated by filtration and washed with 2-propanol. The product was dried in a vacuum oven at 30-40 C. for about 6 hours to afford the sesquisuccinate complex (2.34 g, 85% yield). A 50 mL round bottom flask with magnetic spin bar was charged with <strong>[383432-38-0]E-2-Methoxy-N-(3-{4-[3-methyl-4-(6-methyl-pyridin-3-yloxy)-phenylamino]-quinazolin-6-yl}-allyl)-acetamide</strong> free base (2.0, 1.0 equiv.) and 2-propanol (20 mL). The mixture was heated to solution. A separate 75 mL reactor was charged with succinic acid (0.91 g, 1.8 equivalents) and 2-propanol propanol (10 mL) and warmed to about 45 to 50 C. The free base solution was vacuum filtered to remove any solids and was added to the succinic acid solution over about 10 minutes. The resulting slurry was stirred at 45-50 C. for about 1 hour. The slurry was cooled to 20 C. and stirred overnight. The product was isolated by filtration and washed with 2-propanol. The product was dried in a vacuum oven at 30-40 C. for about 6 hours to afford the sesquisuccinate complex (2.34 g, 85% yield). |
| 85 - 90% |
In water; acetone; at 42 - 58℃;Product distribution / selectivity; |
A suitable clean, dry reaction vessel equipped with programmable linear temperature control system was charged with acetone (130 ml), water (14 ml), succinic acid (7.55 g, 3 eq.), and E-2-Methoxy-N-(3-{4-[3-methyl4-(6-methyl-pyridin-3-yloxy)-phenylamino]-quinazolin-6-yl}-allyl)-acetamide free base (10.0 g, 1 equivalent). The reaction mixture was heated to about 45-58 C. to yield a solution. Once the reaction mixture dissolved in solution, the temperature was adjusted to about 45 C. The solution was vacuum filtered into a suitable clean, dry, speck and fiber-free crystallization vessel. The crystallization vessel was maintained at a jacket temperature of about 50 C. in order to maintain the reaction vessel temperature at about 45 C. The reaction vessel was rinsed with about 20 ml acetone and pressure rinsed through the filter into the crystallization vessel. The reaction mixture was stirred and the temperature of the reaction vessel was adjusted to about 42-45 C. The reaction vessel was seeded with about 1% w/w sesquisuccinate complex. After initiating crystallization by seeding, the reaction mixture was stirred at about 35-45 C. for at least about 1 hour. The vessel was slowly cooled to about 5-20 C., preferably over about 4 hours. The reaction mixture was stirred at about 5-20 C. for about 18 hours. The sesquisuccinate complex was isolated by filtration on a BUchner style funnel, and the cake was washed with acetone at about 20 C. The sesquisuccinate complex was dried to a constant weight by air-drying or in a vacuum oven ranging from about 20-60 C. Sesquisuccinate complex yield: 85-90% w/w.; EXAMPLE 2 To a 500 gallon reactor, E-2-Methoxy-N-(3-{4-[3-methyl-4-(6-methyl-pyridin-3-yloxy)-phenylamino]-quinazolin-6-yl}-allyl)acetamide free base (32.1 kg), succinic acid (24.2 kg), Water (44.9 L), and Acetone (417 L) were added. The mixture was heated to 50 C. and held for 1 hr at 50 C. The solution was filtered to render is speck and fiber free. The solution was collected in second 500 gallon reactor held at about 50 C. The first reactor was rinsed with Acetone (64.2 L) and filtered forward to the speck and fiber free reactor. The solution was cooled to about 40-45 C. over about 30 minutes. Seed crystals of the sesquisuccinate complex (321 g) were added at about 40-45 C. The slurry was held for 2 hours at about 40 C. The slurry was then cooled over about 1 hour to 20 C. and held for about 30 minutes. This was followed by heating back to about 40 C. over one half hour and holding at 40 C. The slurry was cooled over 3 hours to about 35 C., followed by cooling over 2 hours to about 30 C., followed by cooling over 1 hour to 25 C. The slurry was then cooled over about 4 hours to about 0 C. and held at 0 C. for 1 hour. The product was isolated by filtration on a pressurized plate filter covered with a suitable cloth filter media. The product solids were washed with acetone (64.2 L). The product solids were dried for 24 hours at 40 to 50 C. to afford 38.0 kg of the sesquisuccinate complex.; EXAMPLE 9 A suitable clean, dry reaction vessel equipped with programmable linear temperature controls was charged with acetone (130 ml), water (14 ml), succinic acid (7.55 g, 3 eq.), and free base (10.0 g). The amount of succinic acid used in the reaction can range from about 1.5 to 3.5 equivalents. The reaction mixture was stirred at about 45-58 C. to yield a solution. The temperature can be elevated up to the reflux temperature of the solvent to yield a suitable solution. Once the reaction mixture dissolved in solution, the temperature was adjusted to about 45 C. (the temperature should not drop below 42 C.). The solution was filtered in vacuo into a suitable clean, dry, spec-free crystallization vessel. The crystallization vessel was maintained at a jacket temperature of about 50 C. in order to maintain the reaction vessel temperature at about 45 C. The reaction vessel was rinsed with about 20 ml acetone and pressure rinsed through the filter into the crystallization vessel. The reaction mixture was stirred and the temperature of the reaction vessel was adjusted to about 40-45 C. After crystallization begins, the reaction mixture was stirred at about 35-45 C. for at least about 1 hour. The vessel was slowly cooled to about 5-20 C. over about 4 hours. The reaction mixture was stirred at about 20 C. for 18 hours. The sesquisuccinate complex was isolated by filtration on a Buchner funnel, and the cake was washed with acetone. The sesquisuccinate complex was dried to a constant weight using a vacuum oven ranging from about 20-60 C. to afford the sesquisuccinate complex. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping