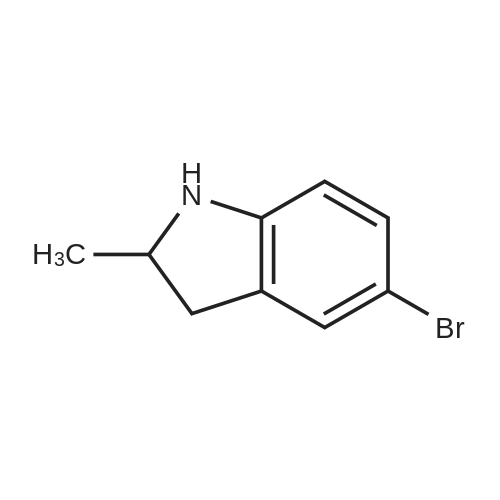
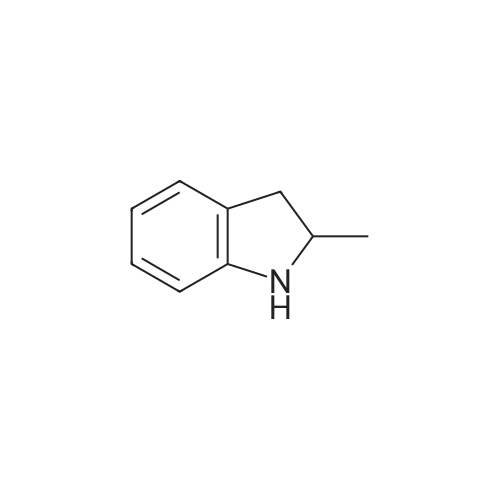
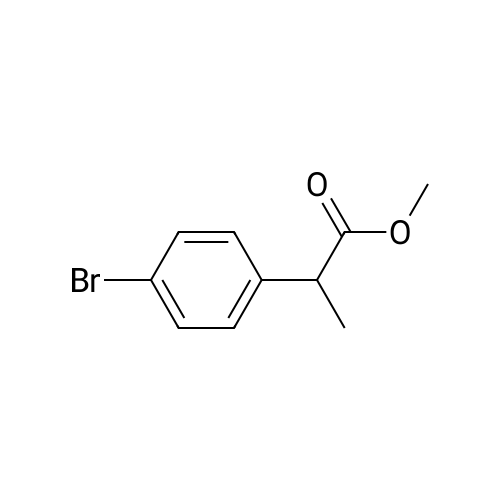
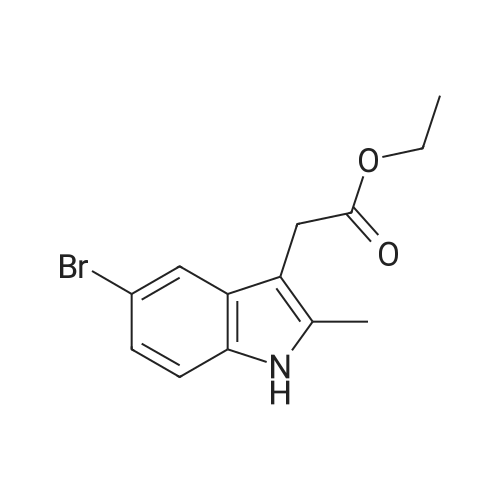
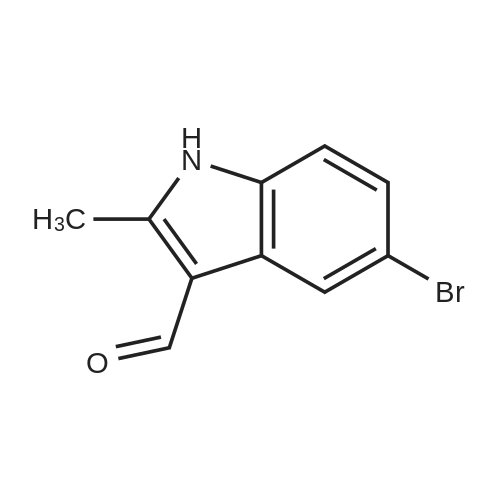
| 68.4% |
With hydrogen; sodium hydride; In DMF (N,N-dimethyl-formamide); for 0.166667h; |
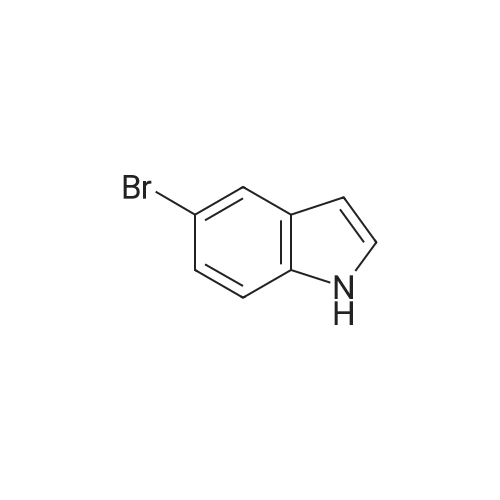
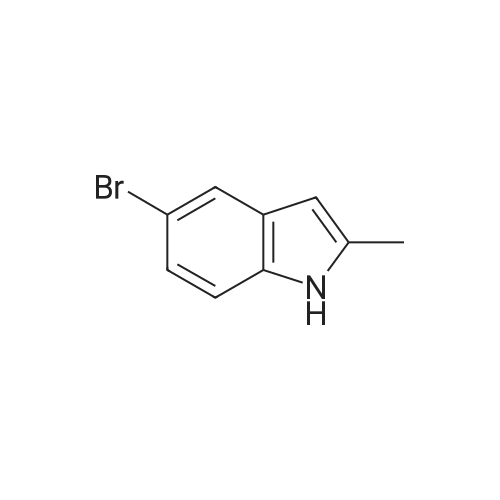
To a solution OF 5-BROMO-2-METHYL-LH-INDOLE (1.5 g, 7.14 mmol) in 10 mL OF DMF was added 60percent sodium hydride in mineral oil (189 mg, 7.9 mmol). Once hydrogen gas evolution ceased, the mixture stirred for 10 minutes and was then treated with 1 RNL (7.83 mmol) of benzene sulfonyl chloride. The reaction was monitored by TLC (ethyl acetate-hexanes (5: 95) ) to afford a major new slightly less polar product than starting material. After 2 hours, the mixture was poured into 50 ML of saturated aqueous ammonium chloride solution and extracted with three 50 mL portions of ethyl acetate. The combined organic layers were washed with four 25 mL portions of brine, dried over magnesium sulfate, treated with activated carbon (NORIT AT"), filtered through diatomaceous earth, and concentrated in vacuo. The residue was adsorbed onto silica gel and chromatographed on silica gel using ethyl acetate-hexanes (1 : 99, then 2: 98, then 3: 97) to afford 1.71 g (68.4percent yield) of 1-BENZENESULFONYL-5-BROMO-2-METHYL-LH-INDOLE as a clear oil. To a chilled solution of L-BENZENESULFONYL-5-BROMO-2-METHYL-LII-INDOLE (350 mg, 0.99 mmol) in 4 mL of THF was added 440 PL (1.10 mmol) of N-BULI (2.5 M solution in hexanes), followed by dimethyl disulfide (100 PL, 1. 11 mmol). The mixture was stirred as it warmed to room temperature and was then quenched with ammonium chloride and extracted with three 15 ML portions of ethyl acetate. The combined organic layers were washed with three 15 mL portions of brine, dried over magnesium sulfate, filtered, and concentrated in vacuo. The crude material was adsorbed onto silica gel and chromatographed on silica gel using ethyl acetate- hexanes (1: 99, then 2: 98) to afford 156 mg (44.3percent yield) of 1-benzenesulfonyl-2-methyl-5- METHYLSULFANYL-LH-INDOLE as a clear oil. To a solution of L-BENZENESULFONYL-2-METHYL-5-METHYLSULFANYL-LH-INDOLE (156 mg, 0.49 mmol) in 10 mL of ethanol was added 10 mL of 10percent aqueous sodium hydroxide solution. The mixture was warmed at reflux for 18 hours. The mixture was then diluted with 10 mL of brine and extracted with three 20 mL portions of ethyl acetate. The combined organic layers were washed with two 10 mL portions of 10percent aqueous sodium hydroxide solution, two 20 ML portions of saturated aqueous ammonium chloride, two 20 mL portions of brine, dried over magnesium sulfate, filtered, and concentrated in vacuo. The crude product was adsorbed onto silica gel and chromatographed on silica gel using ethyl acetate-hexanes (1: 99, then 2: 98, then 3: 97, then 5: 96) to afford 62 mg (71.2percent yield) OF 2-METHYL-5-METHYLSULFANYL-LH-INDOLE |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping