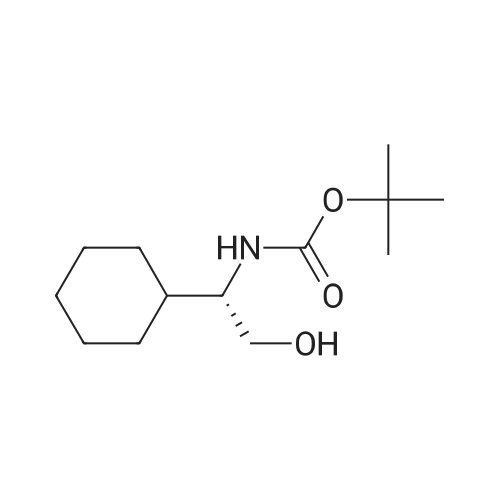
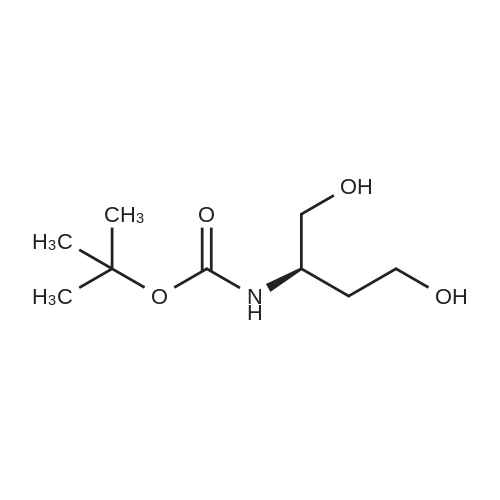
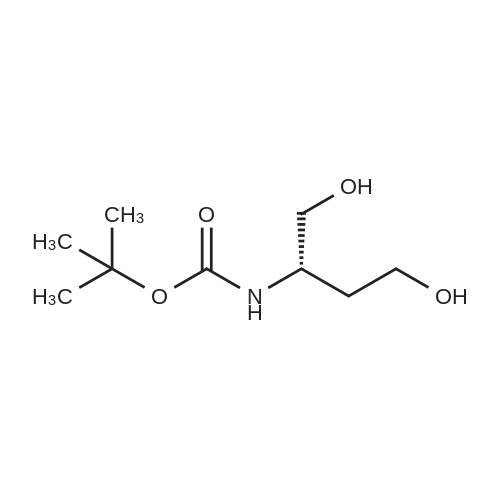
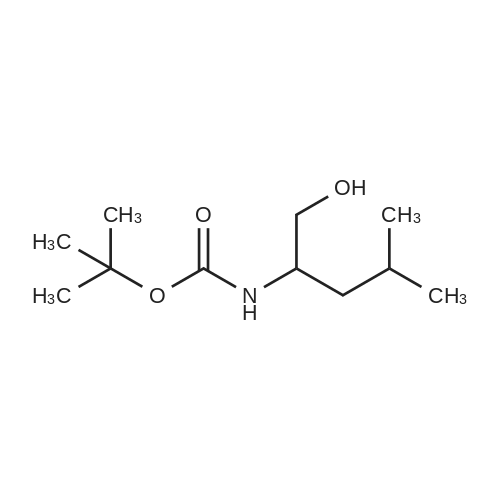
| 90% |
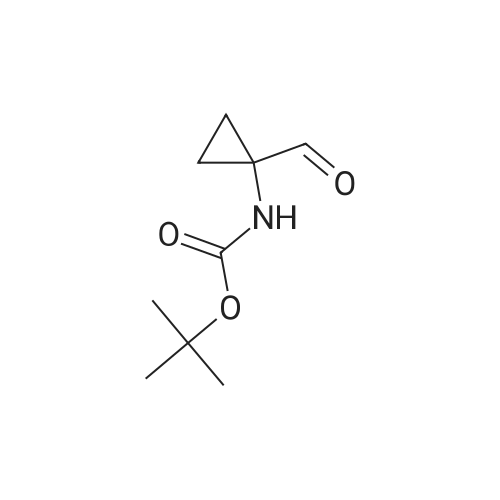
With 4-methylmorpholine N-oxide;tetrapropylammonium perruthennate; In dichloromethane; at 0 - 20℃; for 1h; |
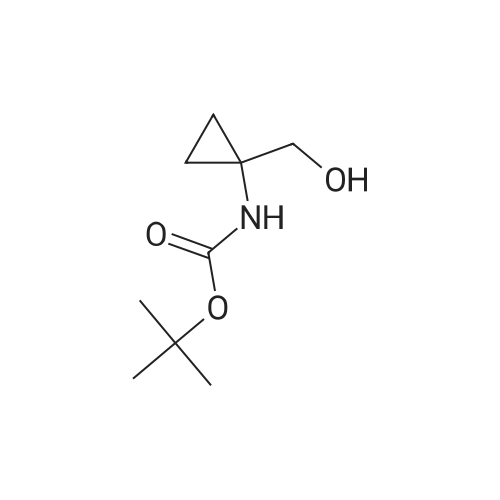
[0505] To a suspension of 1-aminocyclopropanecarboxylic acid (998 mg, 9.87 mmol) in EtOH (25 mL), cooled to 0 C., was added SOCl2 (2.0 mL, 27 mmol) dropwise over 10 minutes. The resulting solution was heated at reflux under nitrogen for 2 hours, then was evaporated under reduced pressure, giving the ester as a light brown oil. [0506] This material was dissolved into EtOAc (25 mL) and a solution of KHCO3 (1.51 g, 15.1 mmol) in H2O (9 mL) was added dropwise. The resulting solution was cooled to 0 C. and a solution of Boc2O (2.97 g, 13.6 mmol) in EtOAc (10 mL) was added. The reaction was stirred at room temperature for 16 hours, the layers were separated and the aqueous solution was extracted with EtOAc (25 mL). The combined organic solution was dried (MgSO4), filtered and concentrated under reduced pressure. Purification by flash column chromatography on silica (EtOAc/hexane, 1:3) gave the protected amine as light brown solid (1.27 g, 5.54 mmol, 56%). 1H NMR (CDCl3) δ 1.08-1.18 (m, 2H), 1.23 (t, 3H, J=7.2 Hz), 1.44 (s, 9H), 1.46-1.53 (m, 2H), 4.14 (q, 2H, J=7.2 Hz), 5.13 (br. s, 1H). [0507] Preparation of (1-hydroxymethyl-cyclopropyl)-carbamic Acid Tert-Butyl Ester: [0508] To a solution of the ester (1.18 g, 5.15 mL) in THF (10 mL) under nitrogen was added a solution of LiBH4 (200 mg, 9.2 mmol) in THF (10 mL) dropwise over 10 minutes. The reaction was stirred at room temperature for 17.5 hours, then was cooled to 0 C. A solution of 50% HOAc was added dropwise until the evolution of gas had ceased (approx. 8 mL). The resulting white suspension was diluted with H2O (15 mL) and was extracted with Et2O (30 mL). The organic solution was washed with 15% aqueous NaHCO3 (15 mL) and brine (15 mL), then dried (Na2SO4), filtered and concentrated under reduced pressure. Purification by flash column chromatography on silica (EtOAc/hexane, 1:1) gave the alcohol as a white solid (592 mg, 3.16 mmol, 61%).2 1H NMR (CDCl3) δ 0.81 (s, 4H), 1.43 (s, 9H), 3.52 (br. s, 1H), 3.58 (s, 2H), 5.12 (br. s, 1H). [0509] Preparation of (1-formyl-cyclopropyl)-carbamic Acid Tert-Butyl Ester: [0510] To a solution of the alcohol (389 mg, 2.08 mmol) in CH2Cl2 (11 mL), cooled to 0 C., was added crushed, dried 3 molecular sieves (1.05 g), NMO (382 mg, 3.26 mmol) and TPAP (76 mg, 0.22 mmol). The black mixture was stirred at 0 C. for 30 minutes and at room temperature for a further 30 minutes. The mixture was diluted with EtOAc (20 mL) and flushed through a short silica column, rinsing with EtOAc. The product containing material was concentrated under reduced pressure giving the aldehyde as a white solid (345 mg, 1.86 mmol, 90%). 1H NMR (CDCl3) δ 1.27-1.37 (m, 2H), 1.40-1.52 (m, 2H), 1.46 (s, 9H), 5.22 (br. s, 1H), 9.16 (s, 1H). [0511] Preparation of (E)-3-(i-tert-butoxycarbonylamino-cyclopropyl)-acrylic Acid Ethyl Ester: [0512] Triethyl phosphonoacetate (0.62 mL, 3.13 mmol) was added dropwise to a suspension of 60% NaH in mineral oil (120 mg, 3.00 mmol) in THF (5 mL). The resulting solution was stirred at room temperature for 10 minutes, then cooled to 0 C. for the dropwise addition of a solution of the aldehyde (463 mg, 2.50 mmol) in THF (5 mL). The reaction was stirred at 0 C. for 15 minutes, then heated to reflux for 1 hour. Once cooled to room temperature, saturated aqueous NH4Cl (10 mL) was added, the layers were separated and the aqueous solution was extracted with CH2Cl2 (10 mL×2). The organic solution was dried (MgSO4), filtered and concentrated under reduced pressure. Purification by flash column chromatography on silica (EtOAc/hexane, 1:1) gave the unsaturated ester as a pale yellow solid (539 mg, 2.11 mmol, 84%). 1H NMR (CDCl3) δ 1.11-1.18 (m, 2H), 1.24-1.29 (m, 5H), 1.44 (s, 9H), 4.17 (q, 2H, J=7.1 Hz), 5.02 (br. s, 1H), 5.84 (d, 1H, J=15.3 Hz), 6.47 (d, 1H, J=15.6 Hz). [0513] Preparation of 3-(1-tert-butoxycarbonylamino-cyclopropyl)-propionic Acid Ethyl Ester: [0514] A solution of the unsaturated ester (495 mg, 1.94 mmol) in EtOAc (10 mL) was hydrogenated (H2 balloon) over 10% Pd/C (25 mg, 0.023 mmol) at room temperature for 3 hours. The mixture was suction filtered through Celite, washing with EtOAc and evaporation of the filtrate under reduced pressure gave the saturated ester as a colourless oil (500 mg, 1.94 mmol, 100%). 1H NMR (CDCl3) δ 0.61-0.65 (m, 1H), 0.73-0.78 (m, 1H), 0.91 (t, 2H, J=7.4 Hz), 1.25 (td, 3H, J=7.1, 1.4 Hz), 1.43 (s, 9H), 1.78-1.90 (m, 2H), 2.36 (t, 1H, J=7.7 Hz), 2.44 (t, 1H, J=7.5 Hz), 4.12 (q, 2H, J=7.1 Hz). [0515] Preparation of [1-(3-hydroxy-propyl)-cyclopropyl]-carbamic Acid Tert-Butyl Ester: [0516] LiBH4 (70 mg, 3.2 mmol) was added to a solution of the ester (500 mg, 1.94 mmol) in THF (8 mL). The reaction was stirred at room temperature under nitrogen for 18 hours, then was quenched by the dropwise addition of 50% aqueous HOAc until the evolution of gas had ceased (approx. 2 mL). The suspension was diluted with H2O (10 mL) and extracted with Et2O (15 mL). The organic solution was washed with saturated aq... |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping