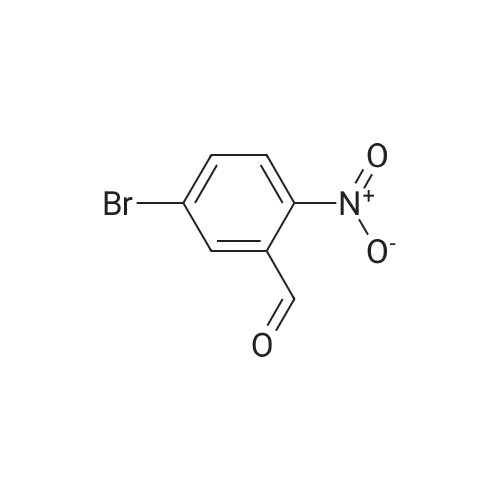
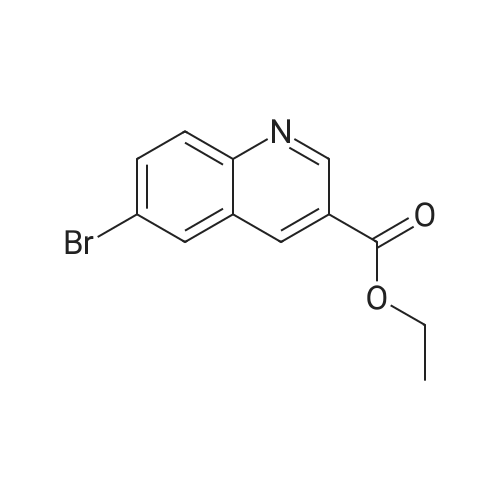
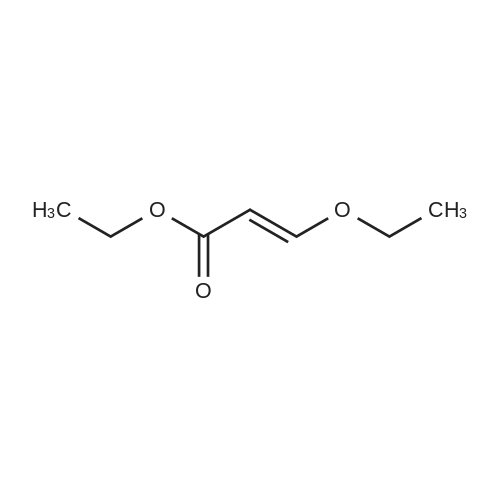
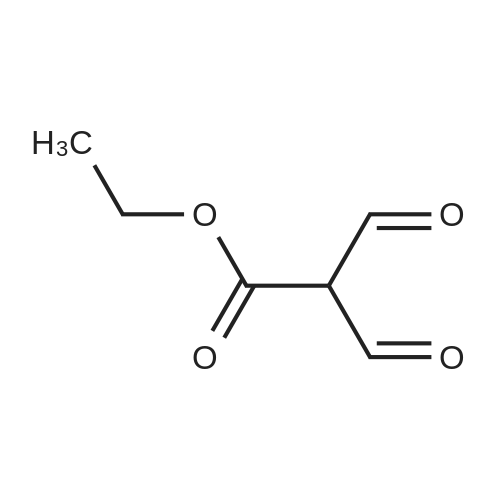
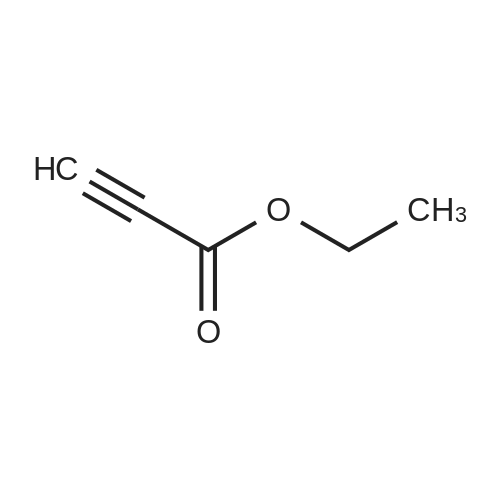
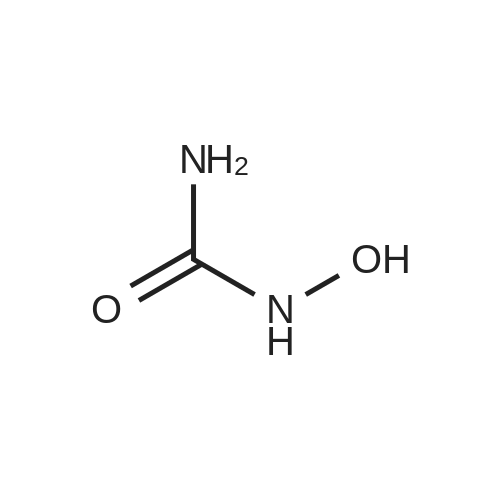
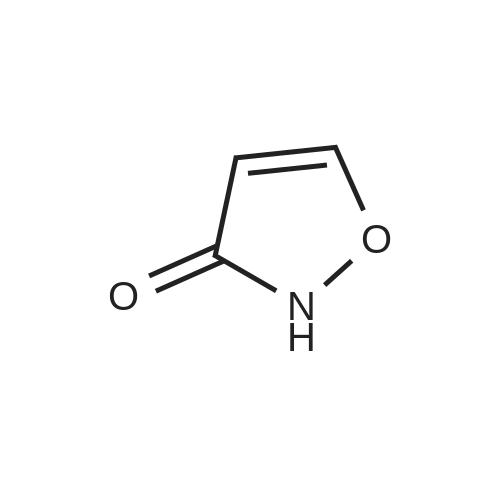
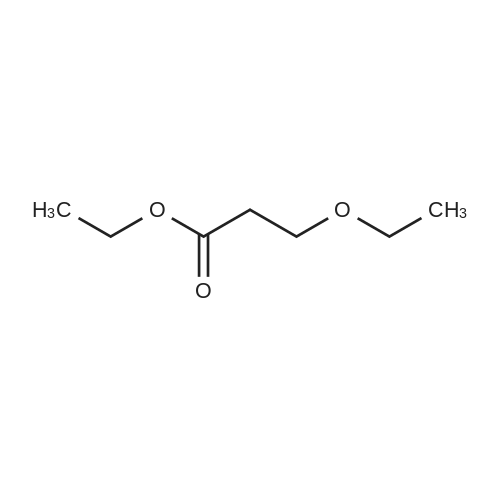
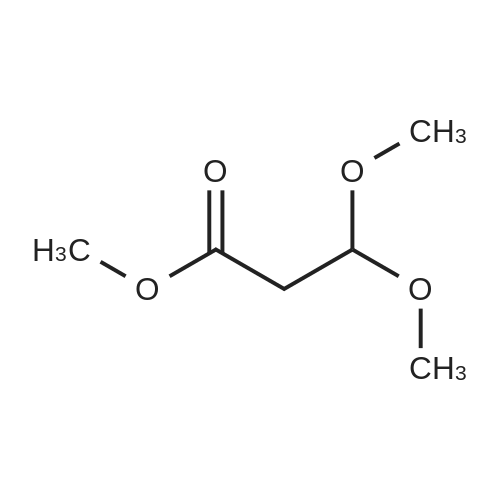
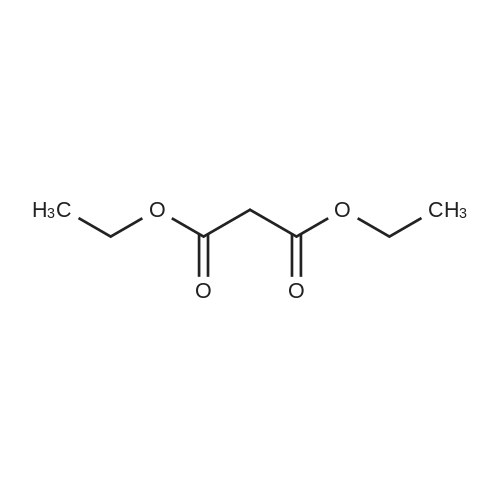
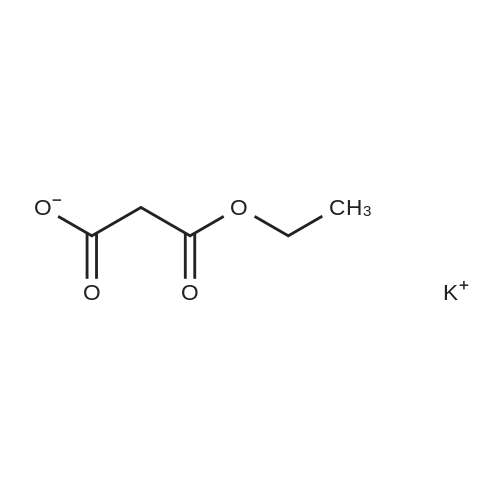
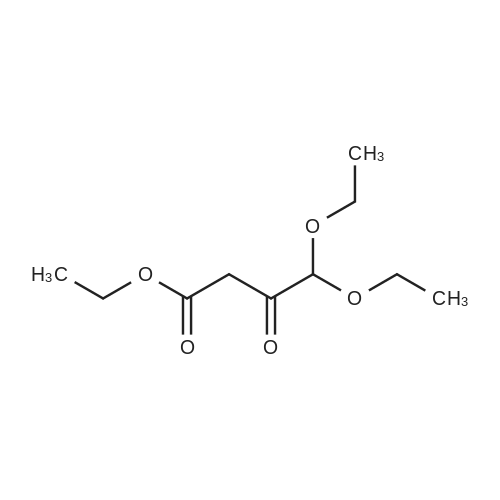
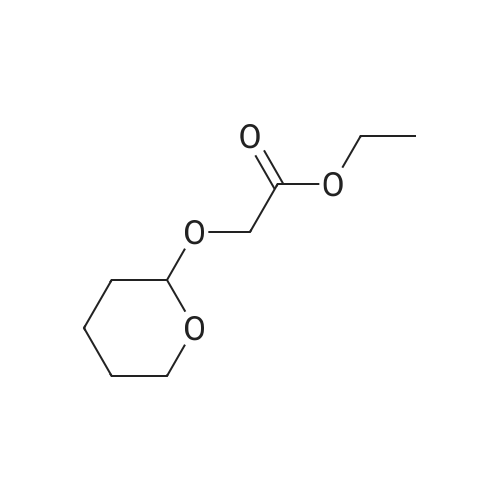
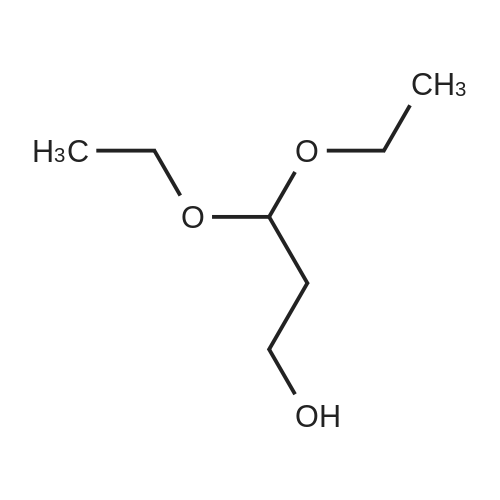
| 74.2% |
With sodium hydride; In diethyl ether; hexane; at 20℃; for 15h;Cooling with ice; |
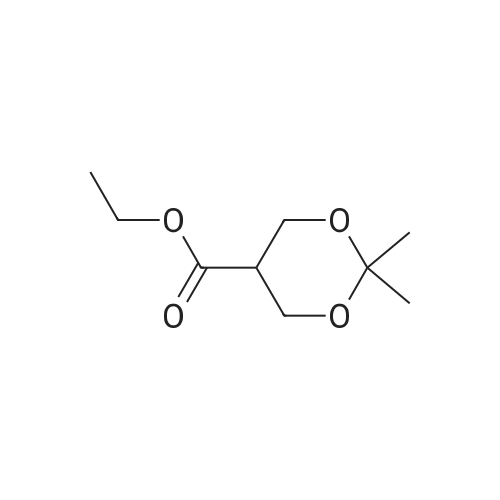
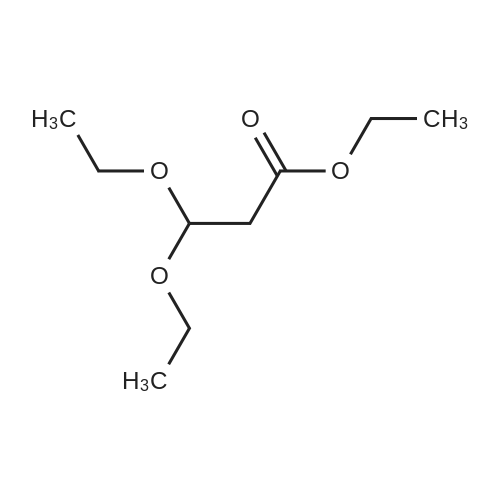
Weighed out sodium hydride (2.46 g, 61.5 mmol) in a dry 100-mL pear flask. Washed with hexanes then with diethyl ether. Suspended in ether (100 mL), cooled in ice bath then ethyl formate (24.84 ml, 308 mmol) was added then ethyl 3,3-diethoxypropanoate (5.98 ml, 30.8 mmol). The resulting mixture was stirred at room temperature for 15 hours to give rise to a reaction. After confirmation of the completion of the reaction, the reaction mixture was poured into water, followed by washing with diethyl ether. The resulting aqueous layer was allowed to have a pH of 1 with hydrochloric acid, followed by extraction with dichloromethane. The resulting organic layer was washed with an aqueous sodium chloride solution and then dried over anhydrous magnesium sulfate, filtered and concentrated to give ethyl 2-formyl-3-oxopropanoate (3.29 g, 22.83 mmol, 74.2 percent yield) as a golden syrup |
| 70% |
|
EXAMPLE 1Preparation of Ethyl-2-formyl-3-oxopropionate[0059] A three- or four-neck round bottom flask equipped with magnetic stir bar, thermocouple, digital thermometer, gas inlet and outlet and addition funnel was flushed <n="19"/>with argon. Ethyl 3,3-diethoxypropionate (64.5 g) in tetrahydrofuran were charged to the addition funnel. Sodium hydride (21.2 g of a 60percent dispersion) was charged to the reaction flask followed by tetrahydrofuran. The contents of the flask were cooled to 0- 5°C in an ice-bath, and ethyl formate (257 g) was added. The mixture was cooled to 0- 50C and the contents of the addition funnel added drop wise, maintaining an internal temperature of less than 5°C. The ice-bath was removed and the contents allowed to warm to ambient temperature. Consumption of ethyl 3,3-diethoxypropionate was monitored by TLC analysis. The reaction was quenched by addition of ice-water (10.6 vol), and extracted three times with methyl t-butyl ether (5.4 vol each), and the organic layers discarded. The aqueous phase was acidified with cone, hydrochloric acid to a pH of 1 to 1.5. The acidified aqueous layer was extracted three times with dichloromethane and the combined organic layers dried over sodium sulfate. The solvent was removed under reduced pressure, and the residue distilled under vacuum, to provide ethyl 2-formyl-3-oxopropionate, 27.92 g, 70percent yield. |
| 70% |
With sodium hydride; In tetrahydrofuran; at 0 - 20℃; |
[0066] A three- or four-neck round bottom flask equipped with magnetic stir bar, thermocouple, digital thermometer, gas inlet and outlet and addition funnel was flushed with argon. Ethyl 3,3- diethoxypropionate (64.5 g) in tetrahydrofuran were charged to the addition funnel. Sodium hydride (21.2 g of a 60percent dispersion) was charged to the reaction flask followed by tetrahydrofuran. The contents of the flask were cooled to 0-50C in an ice-bath, and ethyl formate (257 g) was added. The mixture was cooled to 0-50C and the contents of the addition funnel added dropwise, maintaining an internal temperature of less than 5°C. The ice-bath was removed and the contents allowed to warm to ambient temperature. Consumption of ethyl 3,3- diethoxypropionate was monitored by TLC analysis. The reaction was quenched by addition of ice-water (10.6 vol), and extracted three times with methyl t-butyl ether (5.4 vol each), and the organic layers discarded. The aqueous phase was acidified with cone, hydrochloric acid to a pH of 1 to 1.5. The acidified aqueous layer was extracted three times with dichloromethane and the combined organic layers dried over sodium sulfate. The solvent was removed under reduced pressure, and the residue distilled under vacuum, to provide ethyl 2-formyl-3 -oxopropionate, 27.92 g, 70percent yield. |
| 59% |
With sodium hydride; In tetrahydrofuran; at 0 - 30℃; for 24h; |
4) In a 500 ml four-necked flask equipped with magnetic stirring, 160 ml of tetrahydrofuran and9.84g NaH, mass fraction of NaH in tetrahydrofuran solution is 60percent, the solution is cooled in an ice bath to 0 °C to 5 °C, and then 119g of ethyl formate is added to slowly increase the temperature, when the temperature stabilizes to 0 °C At 5 °C, 80 ml of a tetrahydrofuran solution containing D was continuously added dropwise into a 500 ml four-necked flask, wherein the mass fraction of D was 5percent. After the dropwise addition, the water bath was removed and heated to room temperature. At this time, a large amount of hydrogen was evolved. It will first rise to 30 °C and then gradually decrease to 25 °C. After 24 hours of reaction, the reaction solution was poured into ice water and quenched. 5) The resulting product is first extracted with 500 ml of tert-butyl methyl ether three times to remove the organic phase, and then with concentrated hydrochloric acid to adjust the pH of the aqueous phase to 1~1.5; then extract the aqueous phase with 300 ml of dichloromethane, twice The combined organic phases were combined; the combined organic phases were then dried over anhydrous sodium sulfate and evaporated to give a brown-red liquid which was evaporated in vacuo. GC yield was 99.3percent. The yield was 85.9percent. Finally, the 9 mm Hg diaphragm was distilled under reduced pressure. The colorless transparent liquid E, ethyl 2-formyl-3-oxopropanoate, was obtained in a yield of 59percent. |
|
|
Step 1: 2-Formyl-3-oxo-propionic acid ethyl ester Ethyl 3,3-diethoxypropionate (100 g, 525.7 mmol) was dissolved in THF (360 ml) at room temp. Ethyl formate (175.1 ml, 2.1 mol) was added at room temp. The solution was cooled in an ice-bath to 0° C. and tBuOK (1M solution in THF, 1,156 ml, 1.156 mol) was added via an addition funnel slowly over 30 min, maintaining internal temperature below 5° C. The color changed instantly from colorless to dark orange. The reaction mixture was allowed to warm up to room temp. and stirred for 2 h. The reaction was allowed to stir at room temp. for 18 h. The reaction mixture was concentrated in vacuo and 1 L of solvent was removed. The remaining brownish solution with white solid in it was cooled in an ice-bath and hydrochloric acid (6N, 200 ml) was added to adjust pH=3, maintaining internal temperature below 20° C. The resulting bright yellow suspension was then warmed up to room temp. and stirred for 1 h. Additional 700 ml solvent was removed in vacuo at room temp. Water (400 ml) was added to dissolve all the white solid and ethyl acetate (500 ml) was added and the mixture transferred to a separatory funnel. The aqueous was extracted once with ethyl acetate (200 ml). The combined organic extracts was washed once with brine (100 ml). After drying over MgSO4 and concentrating in vacuo, 2-formyl-3-oxo-propionic acid ethyl ester (75.85 g) was obtained as yellow oil. |
|
With sodium hydride; In tetrahydrofuran; at 0 - 20℃; for 24h; |
Sodium hydride (2.00 g, 50.6 mmol, 60percent) was added to a solution of ethyl formate (10.13 g, 168.7 mmol) in tetrahydrofuran while maintaining the internal temperature below 0 °C. Then, ethyl 3,3-diethoxy propionate (5.00 g, 33.75 mmol) was added dropwise to the reaction mixture and stirred for 24 h at ambient temperature. After confirming that the reaction was complete by using TLC analysis, the reaction was quenched with 20 mL water and the tetrahydrofuran was removed under reduced pressure. The mixture was extracted with ethyl acetate and then brine. The organic phase was dried over Na2SO4 overnight and the solvent was removed in vacuo to give the title compound 14. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping