|
With formic acid; at 20 - 150℃; for 14 - 18h;Product distribution / selectivity; |
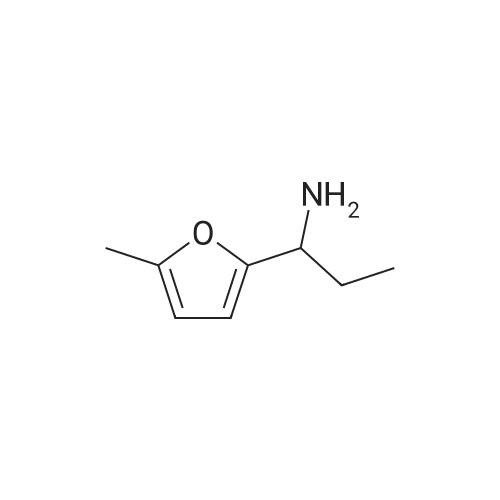
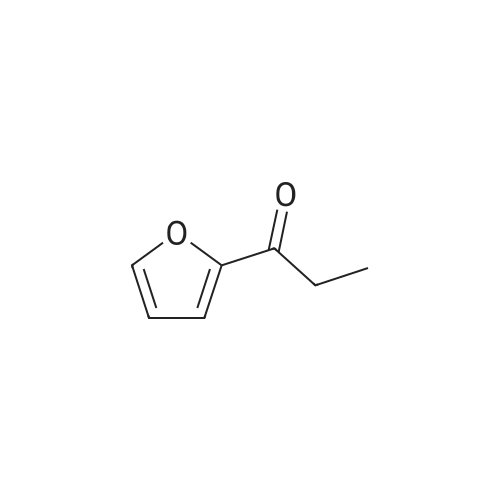
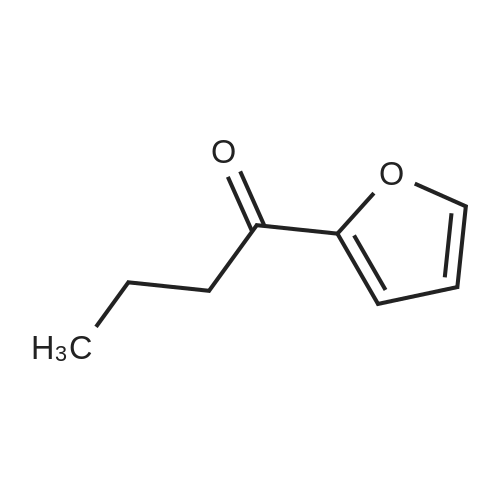
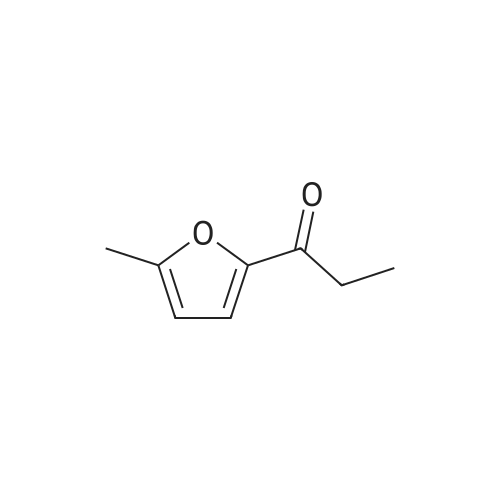
To a stirred solution of 100 g <strong>[10599-69-6]2-methyl-5-propionylfuran</strong> (1.0 equiv., 0.724 mol) and 115 mL formamide (2.90 mol, 4.0 equiv.) at 25 0C was added 30.0 mL formic acid (0.796 mol, 1.1 equiv.). A small exotherm was observed. The resulting solution was heated to 140-150 0C over 1 hour, held at this temperature for 12 hours, and then cooled to 20-30 0C over 1 hour. To the stirred solution of crude intermediate amide product was added 641 mL 25% w/w aq. NaOH (5.07 mol NaOH, 7.0 equiv.). An exotherm was observed. The heterogeneous solution was vigorously agitated to achieve a homogeneous mixture. The solution was heated to 65-70 0C over 30 min., held at this temperature for 10 hours, then cooled to 20-30 0C over 1 hour. The phases were allowed to separate, drained the aqueous layer, then washed the <n="39"/>organic layer of crude racemic amine twice with 10% aq. NaCI (100 ml_). The crude racemic amine was taken up in 350 mL methanol and 28 mL water. The solution was heated to 50-60 0C and to it was added 73.5 g D-tartaric acid (0.502 mol, 1.0 equiv.) as a solution in 210 mL methanol and 14 mL water over 30 minutes. The reaction was held at 60 0C for 15 min, then cooled to 15-35 0C over 2 hours. The suspension was then filtered under vacuum and washed twice with 70 mL methanol. The wet cake was dried in a vacuum oven at 50-60 0C for at least 8 hours to afford 60.1 g (28.7% yield, 99% ee) of a white crystalline solid; mp = 191-1940C; 1H NMR (DMSO-D6): δ 0.81 (t, 3H, J=7.4 Hz), 1.79-1.95 (m, 2H), 2.26 (s, 3H), 3.99 (s, 2H), 4.18 (dd, 1H, J=8.9, 5.7 Hz)1 6.07 (dd, 1H, J=3.1, 1.1 Hz), 6.38 (d, 1H, J=3.1 Hz), and 8.16 (brs, 6H). 13C NMR (DMSO-D6): 10.31, 13.63, 25.46, 49.40, 72.31, 107.03, 109.98, 149.46, 152.01, 175.01 ppm.EXAMPLE IVa Alternative Preparation of tartarate salt of α-(R)-Ethyl-5-methyl-2-furanmethanamine D-tartrate (2DaD2-methy1-5-propionyl furan Intermediate AmideTo a stirred solution of 60 g <strong>[10599-69-6]2-methyl-5-propionylfuran</strong> (1.0 equiv., 0.434 mol) and 69 mL formamide (1.74 mol, 4.0 equiv.) at 25 0C was added 16.4 mL formic acid (0.434 mol, 1.0 equiv.). The resulting solution was heated to 140-150 0C over 1 hour, held at this temperature for 16 hours, and then cooled to 20-30 0C over 1 hour. To the stirred solution of crude intermediate amide product was added 377 mL 25% w/w aq. NaOH (2.89 mol NaOH1 7.0 <n="40"/>equiv.). The heterogeneous solution was vigorously agitated to achieve a homogeneous mixture. The solution was heated to 80-90 0C over 30 min., held at this temperature for 6 hours, then cooled to 20-30 0C over 1 hour. The phases were allowed to separate, and the aqueous layer was drained. The crude racemic amine was distilled under vacuum (20-25 mmHg) to afford 50.1 g (82% yield) of a pale yellow oil; bp = 60-65 0C (40-45 mmHg); 1H NMR (DMSO-D6): δ 0.84 (3H, t, J = 7.4 Hz), 1.49-1.58 (1H, m), 1.61-1.71 (1H, m), 1.61 (2H, brs), 2.21 (3H, s), 3.63 (1H, t, J = 6.54 Hz), 5.93 (1H, dd, J = 2.98, 1.00 Hz), 6.00 (1H, d, J = 1.0 Hz); 13C NMR (DMSO-D6): 10.6, 13.6, 29.7, 51.1, 105.2, 106.1, 149.8, 158.5 ppm. To a solution of the racemic amine in 250 mL methanol was added 50.5 g D-tartaric acid (336.5 mmole) as a solution in 150 mL methanol over 30 minutes. The solution was heated to 40- 50 0C and held at this temperature for 20 minutes. The reaction was slowly cooled to 0-10 0C over 2 hours. The suspension was then filtered under vacuum and washed with methanol (100 mL). The wet cake was dried in a vacuum oven at 50-60 0C for at least 8 hours to afford 44.1 g (42.3% yield from racemic amine, 94% ee) of a white crystalline solid; characterized as above. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping