| 74% |
|
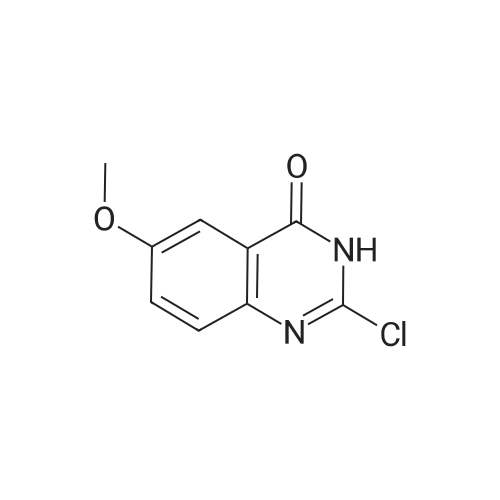
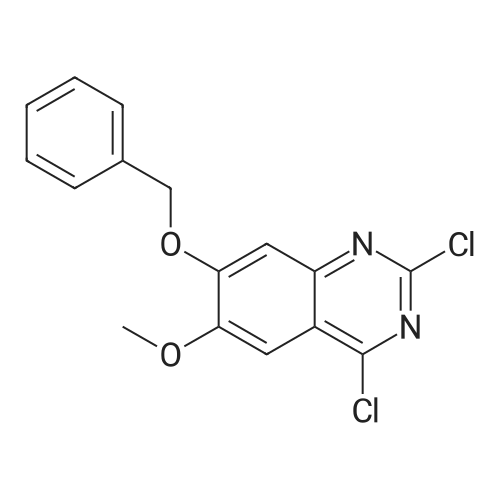
General procedure: A mixture of quinazoline-2,4(1H,3H)-dione 4a (0.48 g, 2.96 mmol) in POCl3 (3.31 mL,35.52 mmol) was stirred at room temperature for 30 min. AfterN,N-diethylaniline (0.167 mL, 0.86 mmol) was added drop-wise,the reaction mixture was heated to reflux for 14 h. After cooling to rt, remained POCl3 was removed under reduced pressure. Thereaction residue was extracted three times with CH2Cl2 (30 mL),dried over MgSO4, concentrated under reduced pressure, and purifiedby column chromatography (EtOAc/n-Hex = 1:9) on silica gelto get the title product 5a in 87% yield (513 mg). |
| 74% |
With N-ethyl-N,N-diisopropylamine; trichlorophosphate; at 125℃; for 3h; |
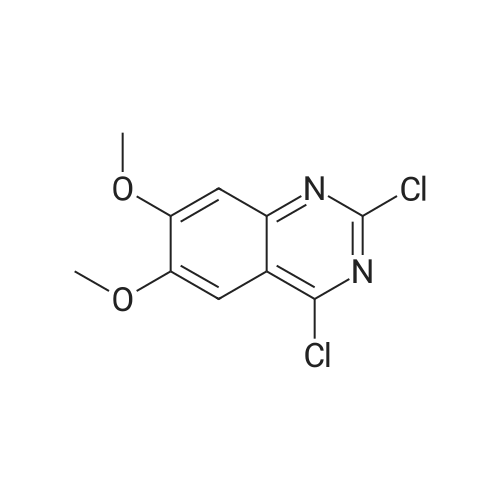
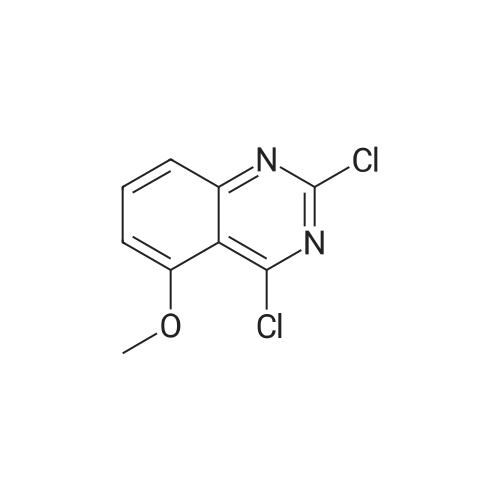
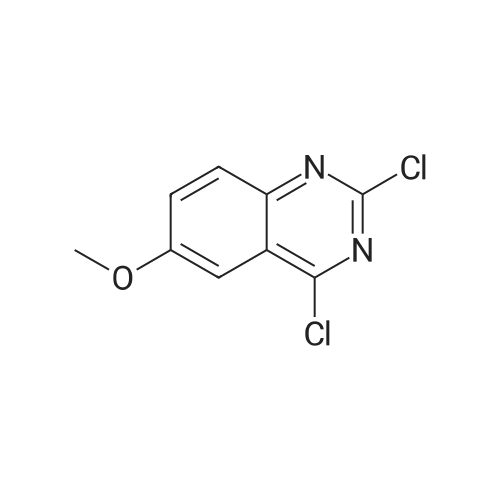
A mixture of 6-methoxyquinazoline-2,4(lH,3H)-dione (1 g, 5.2 mmol) and DIPEA (1.34 g, 10.4 mmol) POCI3 (10 mL) was stirred at 125 C for 3 h. The reaction mixture was cooled to RT, added dropwise to water (100 mL) and extracted with DCM (100 mL*2). The organic layers were dried over Na2S04, filtered and concentrated to afford 2,4-dichloro-6-methoxyquinazoline (880 mg, 74%) as a yellow solid. MS Calcd.: 228 MS Found: 229 ([M+H]+). |
| 69% |
With trichlorophosphate; for 16h;Reflux; |
A solution of 6-methoxyquinazoline- 2,4(lH,3H)-dione (5 g, 26.0 mmol) in trichloro phosphorus oxide (30 mL) was refluxed for 16 hours. After cooling down to ambient temperature, the resulting solution was concentrated under reduced pressure and the residue was purified by silica gel column chromatography, eluted with 1-2% methanol in dichloromethane to afford 2,4-dichloro-6-methoxyquinazoline as a yellow solid: MS (ESI, m/z): 229 A [M + 1]+; 1H MR (300 MHz, CDC13) delta 7.90 (d, J= 9.3 Hz, 1H), 7.61 (dd, J= 2.7 Hz, 6.6 Hz, 1H), 7.41 (d, J= 2.7 Hz, 1H), 4.00 (s, 3H). |
| 28% |
With N,N-diethylaniline; trichlorophosphate; for 6h;Reflux; |
To a solution of 6-methoxyquinazoline-2,4(lH,3H)-dione (5.10 g, 26.6 mmol) in POCI3 (30 mL) was added N,N-diethylaniline (2.4 mL, 15.0 mmol). The mixture was refluxed for 6 hours. The excess POCI3 was removed under reduced pressure and the residue was dissolved in EtOAc (100 mL), the organic layer was washed with sat.NaHCC>3 (30 mL), dried over Na2S04, filtered and concentrated to give the crude product which was purified by silica gel column (PE / EtOAc=20 / 1) to give the product (1.70 g, yield 28%). |
| 3.6 g |
With dimethyl amine; trichlorophosphate; at 110℃; |
<Step 2> 2,4-dichloro-6-methoxyquinazoline N,N-dimethylamine (6.3 mL, 50 mmol) was added into a mixed solution of 6-methoxyquinazolin-2,4(1H,3H)-dione (4.8 g, 25.0 mmol) prepared in Step 1 in phosphorus oxychloride (50 mL), and then they were stirred at 110 C. overnight. After cooling to room temperature, the reaction mixture was added into ice water and then basified to pH 9 with sodium hydroxide. The aqueous layer was extracted with ethyl acetate, and the organic layer was dried on anhydrous sodium sulfate and concentrated under reduced pressure. The resulting residue was purified with silica gel column chromatography (n-hexane/ethyl acetate=5/1) to give the titled compound (3.6 g) as a yellow solid. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping