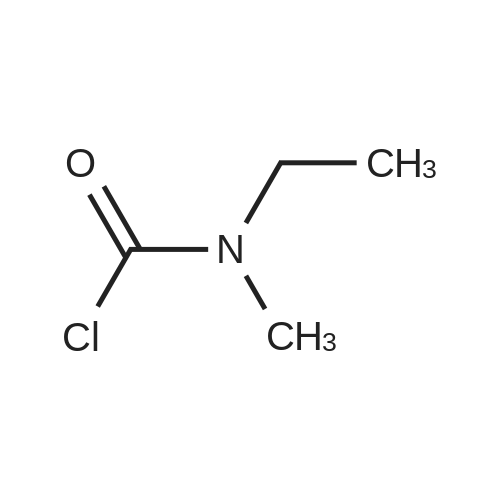
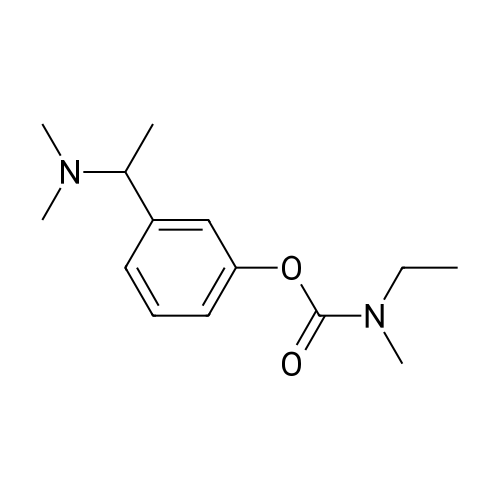
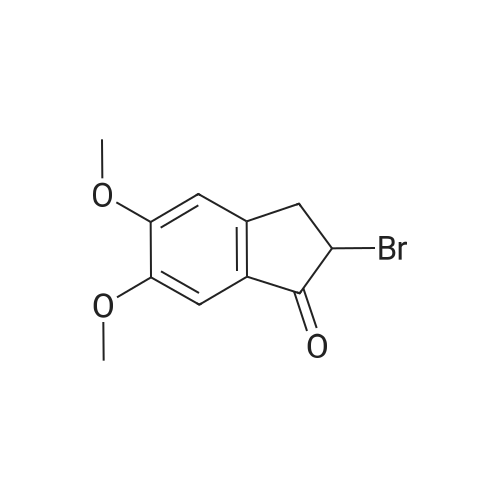
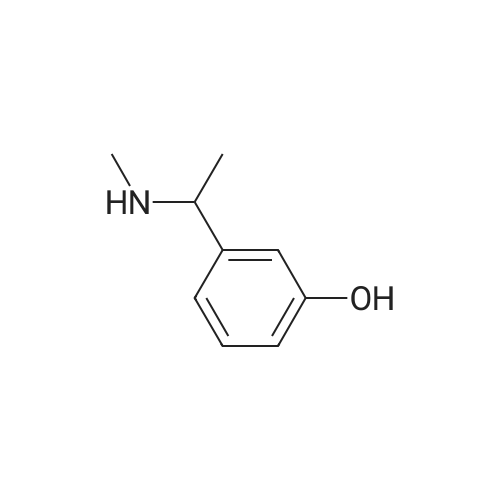
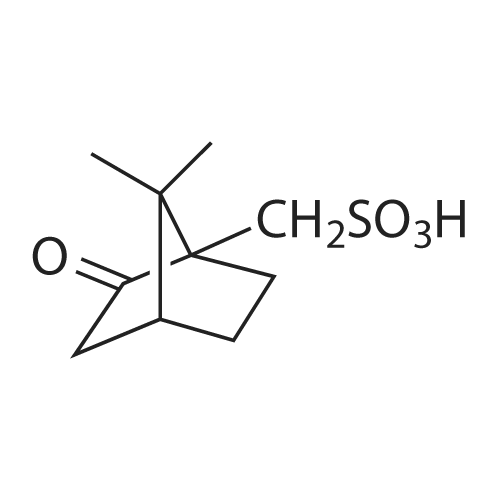
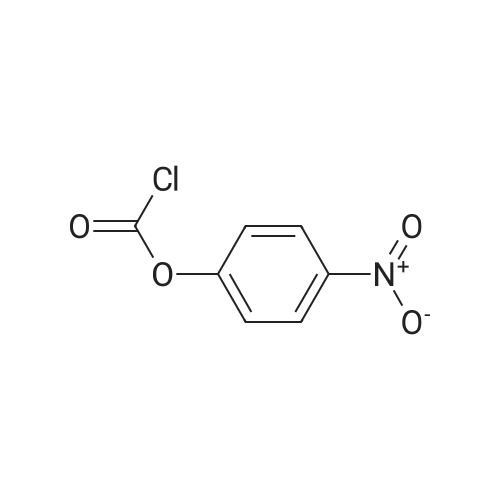
| 85% |
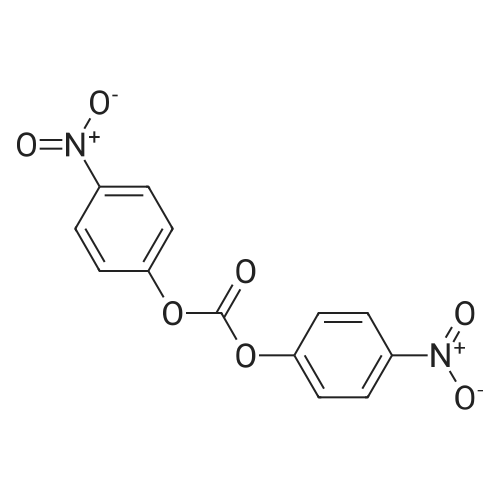
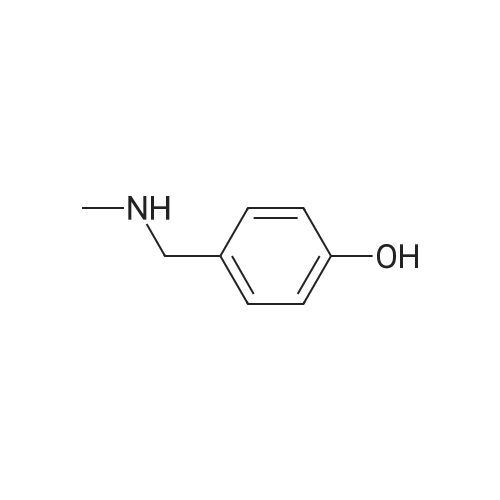
With hydrogen;nickel; In methanol; at 80℃; under 7355.72 Torr; for 3 - 4h;Product distribution / selectivity; |
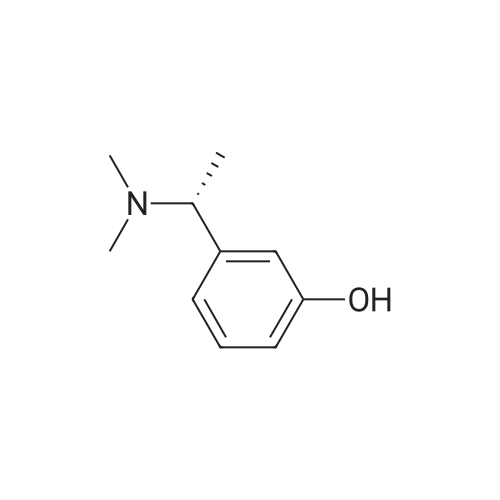
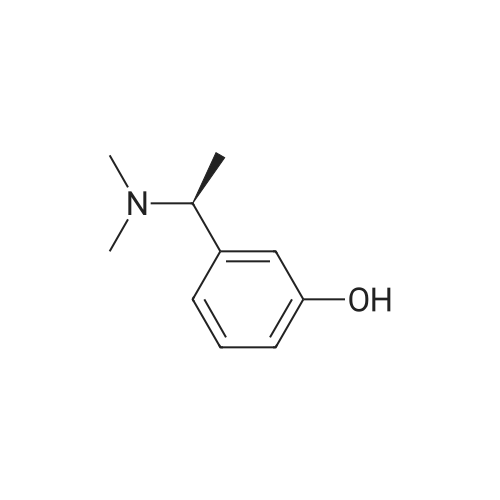
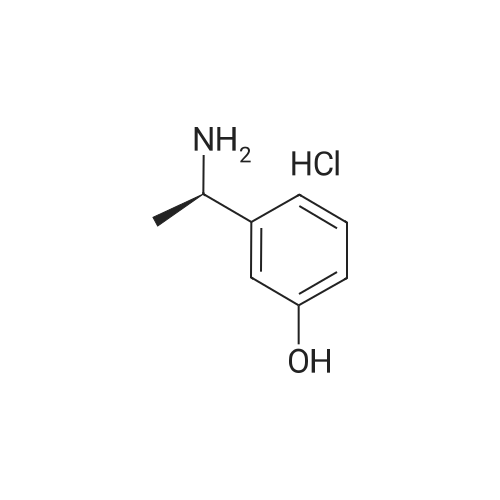
Example 2: Preparation of alpha-m-hydroxy phenylethyldimethylamine; N-methylation was carried out on alpha-m-hydroxy phenylethylmethylamine (25g) with Paraformaldehyde (15g) in presence of Raney Nickel (3Og) in methanol(500ml) at 80C and 10kg /cm2 of lrydrogen pressure in an autoclave. After 3-4 hours the product was isolated by removing Raney nickel and concentrating the filtrate. The product was further <n="9"/>purified by dissolving the crude product in Toluene (50ml) and is crystallized by slow addition of Petroleum ether (150ml). Pure alpha-m-hydroxy phenylethyldimethylarnine is isolated (23g, Yield: 85%, purity 98%) by filtration.Characterization data:1H-NMR (DMSO): 9.25(1H, s), 7.05(1H, t), 6.60(3H, m), 3.08(1H5 q), 2.05(6H, s),1.19(3H, d)13C-NMR (DMSO): 158.0, 146.7, 129.8, 118.7, 114.8, 114.5, 65.8, 43.6, 20.9.Mass(Methanol) : 166.2 (M+l); Example 4: Preparation of alpha-m-hydroxy phenylethyldimethylamine; N-methylation was carried out on alpha-m-hydroxy phenylethylmethylamine (25g) with Paraformaldehyde (15g) in presence of Raney Nickel (30g) in methanol (500ml) at 80C and 10kg /cm of hydrogen pressure in an autoclave. After 3-4 hours the product was isolated by removing Raney nickel and concentrating the filtrate. The product was further purified by dissolving the crude product in Toluene (50ml) and is crystallized by slow addition of Petroleum ether (150ml). Pure alpha-m-hydroxy phenylethyldimethylamine is isolated (23g, Yield: 85%, purity 98%) by filtration.Characterization data: 1H-NMR (DMSO): 9.25(1H, s), 7.05(1H, t), 6.60(3H, m), 3.08(1H, q), 2.05(6H, s), 1.19(3H, d) 13C-NMR (DMSO): 158.0, 146.7, 129.8, 118.7, 114.8, 114.5, 65.8, 43.6, 20.9. <n="10"/>Mass(Methanol) : 166.2 (M+l) |
| 85% |
With hydrogen;Raney nickel; In methanol; at 80℃; under 7355.72 Torr; for 3 - 4h;Product distribution / selectivity; |
Example 2 Preparation of alpha-m-hydroxy phenylethyldimethylamine N-methylation was carried out on alpha-m-hydroxy phenylethylmethylamine (25 g) with Paraformaldehyde (15 g) in presence of Raney Nickel (30 g) in methanol (500 ml) at 80 C. and 10 kg/cm2 of hydrogen pressure in an autoclave. After 3-4 hours the product was isolated by removing Raney nickel and concentrating the filtrate. The product was further purified by dissolving the crude product in Toluene (50 ml) and is crystallized by slow addition of Petroleum ether (150 ml). Pure alpha-m-hydroxy phenylethyldimethylamine is isolated (23 g, Yield: 85%, purity 98%) by filtration. Characterization Data: 1H-NMR (DMSO): 9.25(1H, s), 7.05(1H, t), 6.60(3H, m), 3.08(1H, q), 2.05(6H, s), 1.19(3H, d) 13C-NMR (DMSO): 158.0, 146.7, 129.8, 118.7, 114.8, 114.5, 65.8, 43.6, 20.9. Mass (Methanol): 166.2 (M+1) Example 4Preparation of alpha-m-hydroxy phenylethyldimethylamineN-methylation was carried out on alpha-m-hydroxy phenylethylmethylamine (25 g) with Paraformaldehyde (15 g) in presence of Raney Nickel (30 g) in methanol (500 ml) at 80 C. and 10 kg/cm2 of hydrogen pressure in an autoclave. After 3-4 hours the product was isolated by removing Raney nickel and concentrating the filtrate. The product was further purified by dissolving the crude product in Toluene (50 ml) and is crystallized by slow addition of Petroleum ether (150 ml). Pure alpha-m-hydroxy phenylethyldimethylamine is isolated (23 g, Yield: 85%, purity 98%) by filtration.Characterization Data:1H-NMR (DMSO): 9.25(1H, s), 7.05(1H, t), 6.60(3H, m), 3.08(1H, q), 2.05(6H, s), 1.19(3H, d)13C-NMR (DMSO): 158.0, 146.7, 129.8, 118.7, 114.8, 114.5, 65.8, 43.6, 20.9.Mass (Methanol): 166.2 (M+1) |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +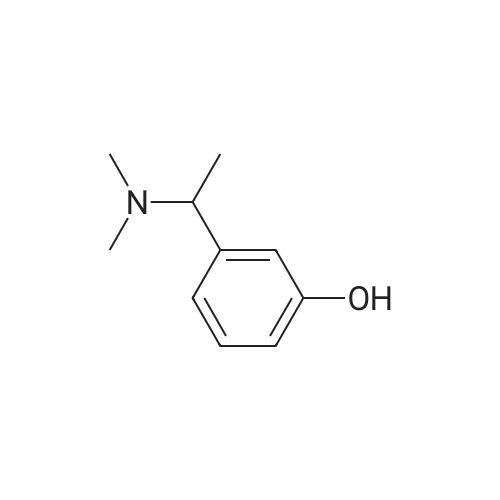

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping