| 71% |
With dmap; triethylamine; In dichloromethane; at 0 - 5℃; for 2h; |
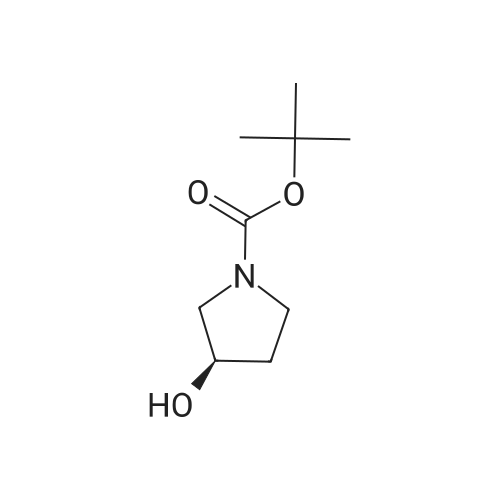
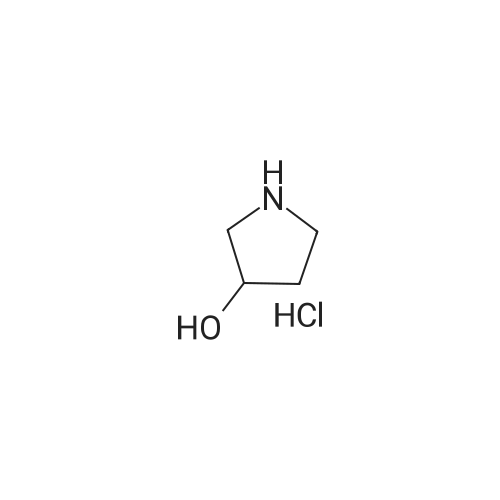
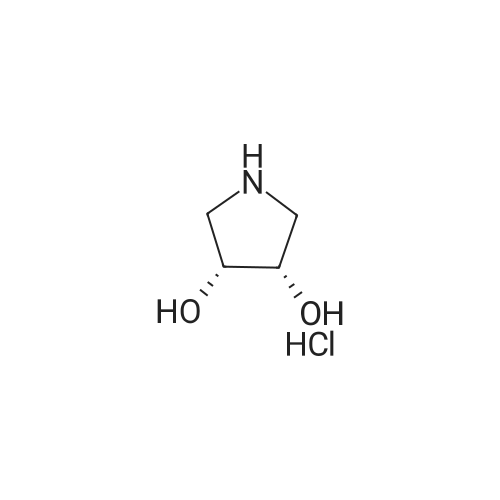
Step 2: Preparation of (3R)-l-(tert-butoxycarbonyl)-3-hydroxypyrrolidine (IX): To a stirred suspension of 3-(i?)-hydroxypyrrolidine hydrochloride (VIII) (110 g, 0.9 mol) in dichloromethane (1100 ml), triethylamine (273 g, 2.7 mol) was added at 0- 5C. After 5 minute of stirring di-feri-butyldicarbonate [(Boc)20] (245 g, 1.125 mol) was added to the reaction mixture in small portions, followed by 4-dimethylaminopyridine (10.99 g, 0.09 mol). The reaction mixture was stirred for 2 hour and then poured in to water (1100 ml). The organic layer was separated and washed with saturated ammonium chloride solution (1x1100 ml) and water (1100 ml). The organic layer was dried over anhydrous sodium sulphate and the solvent evaporated under reduced pressure. The residue was purified by silica gel (60-120 mesh) column chromatography using 1-5% mixtures of acetone: hexane as an eluent. The combined fractions were evaporated, to obtain the 118 g of (3i?)-l-(ieri-butoxycarbonyl)-3-hydroxypyrrolidine (IX), as a white solid, in 71 % yield. Analysis: Melting point: 55 - 58C; Mass: 188 (M+l); for Molecular Weight of 187.24 and Molecular Formula of C9H17N03; and 1H NMR (400MHz, CDC13): 54.428 - 4.424 (s, 1H), 3.46 - 3.43 (m, 2H), 3.37 - 3.28 (m, 2H), 2.36 - 2.30 (d, 1H), 2.00 - 1.86 (m, 2H), 1.44 (s, 9H). |
| 70% |
With triethylamine; In methanol; at 0 - 20℃; |
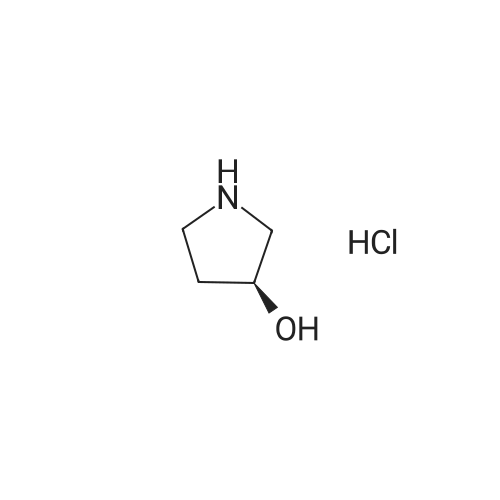
Di-tert-butyl dicarbonate (1.6 g, 7.3 mmol) was added to a solution of (R)-3-pyrrolidinol hydrochloride (1.0 g, 8.1 mmol) in MeOH (20 mL) and triethylamine (3.4 mL, 24.3 mmol) at 0 C. The reaction was stirred overnight while warming to rt. Solvent was removed in vacuo. The residue was diluted with EtOAc (50 mL), washed with water (40 mL*3), washed with brine (50 mL), dried (Na2SO4), and concentrated to give 950 mg (70%) of the title compound as a white solid. 1H NMR (400 MHz, DMSO-d6): δ 1.37 (9H, s), 1.60-1.90 (2H, m), 3.00-3.30 (4H, m), 4.19 (1H, m), 4.87 (1H, d, J=2.8 Hz). |
|
With dmap; triethylamine; In dichloromethane; at 20℃; for 2.25h; |
Solid di-tert-butyldicarbonate (38.8g, 178mmol) was added in portions over 15 minutes to a stirred solution of (3R)-pyrrolidin-3-ol hydrochloride (20g, 162mmol), triethylamine (24.8mL, 178MMOL) and 4- (DIMETHYLAMINO)-PYRIDINE (DMAP) (20mg) in dry dichloromethane (300mL). After stirring for 2 hours at room temperature, the mixture was washed with aqueous citric acid, then brine. The organic extracts were dried (MGS04), filtered and evaporated in vacuo to give an oil. This was purified by flash chromatography on silica, eluting with ethyl acetate/cyclohexane (20: 80 to 60: 40), to give the title compound as a solid |
|
With dmap; In dichloromethane; at 20℃; for 2h; |
Solid ditert-butyldicarbonate (38. 8G, 178mmol) was added in portions over 15 minutes to a stirred solution of (3R)-pyrrolidin-3-ol hydrochloride (20g, 162MMOL), triethylamine (24. 8mL, 178mmol) and 4- (dimethylamino)-pyridine (20mg) in dry dichloromethane (300mL). After stirring for 2 hours at room temperature, the mixture was washed with aqueous citric acid, then brine. The organic extracts were dried (MgSO4), filtered and evaporated in vacuo to give an oil. This was purified by flash chromatography on silica, eluting with ethyl acetate/cyclohexane (20: 80 to 60: 40), to give the title compound as a solid. |
|
With dmap; triethylamine; In dichloromethane; at 20℃; for 2.25h; |
Solid ditert-butyldicarbonate (38.8g, 178mmol) was added in portions over 15 minutes to a stirred solution of (3R)-pynolidin-3-ol hydrochloride (20g, 162mmol), triethylamine (24.8mL, 178mmol) and 4- (DIMETHYLAMINO)-PYRIDINE (20mg) in dry dichloromethane (300mL). After stirring for 2 hours at room temperature, the mixture was washed with aqueous citric acid, then brine. The organic extracts were dried (MGS04), filtered and evaporated in vacuo to give an oil. This was purified by flash chromatography on silica, eluting with ethyl acetate/cyclohexane (20: 80 to 60: 40), to give the title compound as a solid. |
|
With dmap; triethylamine; In dichloromethane; at 20℃; for 2.25h; |
Solid ditert-butyldicarbonate (38.8g, 178mmol) was added in portions over 15 minutes to a stirred solution of (3R)-pyrrolidin-3-ol hydrochloride (20g, 162mmol), triethylamine (24. 8mL, 178mmol) and 4- (dimethylamino)-pyridine (20mg) in dry dichloromethane (300mL). After stirring for 2 hours at room temperature, the mixture was washed with aqueous citric acid, then brine. The organic extracts were dried (MgS04), filtered and evaporated in vacuo to give an oil. This was purified by flash chromatography on silica, eluting with ethyl acetate/cyclohexane (20: 80 to 60: 40), to give the title compound as a solid. |
|
With sodium hydrogencarbonate; In tetrahydrofuran; at 20℃; for 1.5h; |
Boc2O (1.02 mL, 4.5 mmol) was added to a solution of (R)-3-hydroxylpyrrolidine hydrochloride (R)-2a·HCl (0.50 g, 4.1 mmol) in THF-satd NaHCO3 (1:1, 20 mL), and the reaction mixture was stirred at rt for 1.5 h. EtOAc was added, and the layers were separated. The aqueous layer was extracted three times with EtOAc. The combined organic layer was washed with brine, dried over Na2SO4, filtered, and concentrated in vacuo to give tert-butyl (R)-3-hydoxypyrrolidine-1-carboxylate, which was used for the following reaction without further purification.The above-described tert-butyl (R)-3-hydoxypyrrolidine-1-carboxylate was dissolved in anhydrous DMF (20 mL), to which was added NaH (55% oil suspension, 0.71 g, 16.2 mmol) at 0 C. The ice-cold reaction mixture was stirred for 30 min, and Me2SO4 (0.77 mL, 8.1 mmol) was then added. The reaction mixture was stirred overnight at 50 C before being quenched with water. Hexane-EtOAc (1:1) was added, the layers were separated, and the aqueous layer was extracted three times with hexane-EtOAc (1:1). The combined organic layer was washed two times with brine, dried over Na2SO4, filtered, and concentrated in vacuo. The residue was purified by flash column chromatography (hexane-EtOAc, 2:1) to afford 0.69 g of tert-butyl (R)-3-methoxypyrrolidine-1-carboxylate [85% from (R)-2a·HCl]. A colorless oil, -8.4 (c=0.52, CHCl3). 1H NMR (500 MHz, CDCl3) δ: 1.44 (9H, s), 1.84-2.02 (2H, m), 3.31 (3H, s), 3.34-3.49 (4H, m), 3.91 (1H, brs). 13C NMR (125 MHz, CDCl3) δ: 28.5, 30.0, 31.1, 43.5, 43.9, 50.3, 51.1, 56.5, 79.09, 79.14, 79.9, 154.5, 154.6. IR (CHCl3): 1686, 1416 cm-1. HRMS Calcd for C10H19NNaO3 [(M+Na)+] m/z: 224.1257, found: 224.1248.Under a nitrogen atmosphere, 4 M HCl in EtOAc (1.2 mL) was added to tert-butyl (R)-3-methoxypyrrolidine-1-carboxylate (50 mg, 0.25 mmol) at 0 C. The solution was stirred at rt for 30 min and concentrated in vacuo. The residue was dissolved in MeCN-water (10:1, 2.5 mL). Aqueous NH3 (30% w/w, 35 μL, 0.62 mmol) and 3 (162 mg, 0.62 mmol) were added to the solution at 0 C. The reaction mixture was stirred at rt for 30 min and concentrated in vacuo, and the residue was purified by flash column chromatography (CH2Cl2-MeOH, 15:1→10:1) to give 21 mg of (R)-1d (75%, 99% ee) and 7.1 mg of (R)-4-methoxy-1-pyrroline N-oxide (R)-4d (25%). The optical purity of (R)-1d was determined by Daicel CHIRALPAK AD-3 [hexane-iPrOH, 95:5, 2.0 mL/min; retention times 20.3 (R), 24.6 min (S)].(R)-1d. Pale yellow oil, +113 (c=0.85, CHCl3). 1H NMR (500 MHz, CDCl3) δ: 2.17 (1H, dddd, J=3.5, 5.0, 9.0, 14.5 Hz), 2.48-2.57 (1H, m), 3.35 (3H, s), 3.87 (1H, dddd, J=1.0, 6.5, 9.0, 15.5 Hz), 4.10-4.19 (1H, m), 4.56-4.61 (1H, m), 7.02 (1H, q, J=1.5 Hz). 13C NMR (125 MHz, CDCl3) δ: 27.0, 56.5, 61.4, 80.0, 133.3. IR (CHCl3): 1584, 1269, 1238 cm-1. HRMS Calcd for C5H9NNaO2 [(M+Na)+] m/z: 138.0526, found: 138.0534.(R)-4-Methoxy-1-pyrroline N-oxide [(R)-4d]. A pale yellow oil, -22.5 (c=0.66, CHCl3). 1H NMR (500 MHz, CDCl3) δ: 2.75 (1H, d, J=19.5 Hz), 2.94-3.03 (1H, m), 3.33 (3H, s), 3.94 (1H, d, J=15.0 Hz), 4.08-4.15 (1H, m), 4.19-4.24 (1H, m), 6.84-6.87 (1H, m). 13C NMR (125 MHz, CDCl3) δ: 36.1, 56.5, 67.3, 74.3, 133.1. IR (CHCl3): 1595, 1275, 1238 cm-1. HRMS Calcd for C5H9NNaO2 [(M+Na)+] m/z: 138.0526, found: 138.0533. |
| 1.89 g |
With triethylamine; In methanol; at 20℃;Cooling with ice; |
To a solution of (R)-pyrrolidin-3-ol HC1 salt (1.23 g, 10.0 mmol) and Et3N (2.20 g, 20.0 mmol) in MeOH (20 mL) was added (Boc)20(2.20 g, 10.1 mmol) at ice bath. The mixture was stirred at rt overnight. The solvent was removed and the residue was extracted with DCM. The organic layers were washed with brine (20 mL x 2), dried over Na2SO4 and concentrated to give the title product (1.89 g) as a colorless oil which was used directly in the next step. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping