| 1.76 g |
With sodium hydroxide; In 1,4-dioxane; water; at 0 - 20℃; for 24h; |
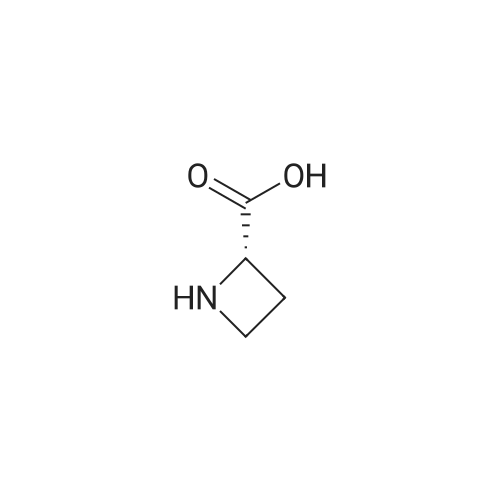
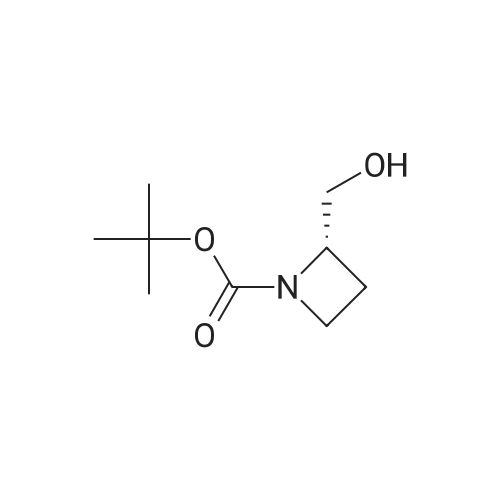
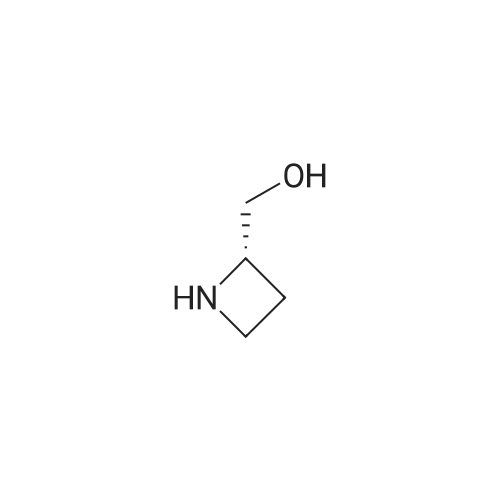
Borane tetrahydrofuran complex (1.0M solution in tetrahydrofuran,25.0 mL, 24.7 mmol) was added to a solution of L-azetidine-2-carboxtlicacid (5, 1.0 g, 9.9 mmol) in tetrahydrofuran (15.0 mL) under nitrogenatmospheric conditions. The resulting mixture was stirred for 90 minunder reflux. Afetr the reaction had cooled to room temperature, anaqueous solution of potassium hydrogen sulfate (10%, 15.0 mL) wasadded to the mixture, which was further refluxed for 15 min. Themixture was evaporated under reduced pressure to yield the residue of(S)-azetidine-2-ylmethanol. An aqueous solution of sodium hydroxide(1 M, 20.0 mL) and di-tert-butyl dicarbonate (2.4 g, 11.9 mmol) wereadded to a solution of (S)-azetidine-2-ylmethanol (862 mg, 9.9 mmol)in water (20.0 mL) and 1,4-dioxane (15.0 mL) at 0 C. The mixture wasthen stirred for 24 h at room temperature. The reaction mixture waspoured into a saturated aqueous solution of sodium bicarbonate andextracted with chloroform. The organic layer was dried over sodiumsulfate and concentrated under reduced pressure. The residue waspurified by silica gel column chromatography (n-hexane/ethylacetate=1/1) to yield (-)-6 (1.79 g, 97%) in the form of a colorless oil: 1H NMR (300 MHz, CDCl3) δ: 4.42 (br s, 1H), 3.91-3.73 (m, 4H),2.32 (br s, 1H), 2.24-2.13 (m, 1H), 1.96 (brs, 1H), 1.45 (s, 9H); 13CNMR (75 MHz, CDCl3) δ: 157.1, 80.1, 66.4, 63.5, 46.6, 28.2 (3), 17.87;IR (CHCl3) cm-1: 3400, 3005, 2978, 1666, 1414, 1369; FAB-MS m/z:188.1282 (Calcd for C9H18NO3: 188.1287); MS (FAB) m/z:188(M++H, 31); [α]D20=-20.59 (c=1.19, CHCl3). |
| 1.79 g |
With sodium hydroxide; In 1,4-dioxane; water; at 0 - 20℃;Inert atmosphere; |
To a solution of L-azetidine-2-carboxylic acid (1.0 g, 9.9 mmol, >99% e.e.) in THF (15 mL), a borane-THF complex (1.0 M solution in THF, 25 mmoL) was added. The mixture was refluxed under a N2 stream for 1.5 hr. Potassium hydrogen sulfate (10% solution in water,15 mL) was added to the mixture, and then the mixture was refluxed for15 min. Solvent was removed under reduced pressure to afford a white solid. Water (20 mL), 1,4-dioxane (15 mL), sodium hydroxide (1M solution in water, 20 mL), and di-tert-butyl dicarbonate (2.4 g, 11.9 mmol) at 0 C were added to the residue. The mixture was stirred at 0 C for 30 min and then warmed to room temperature and stirred overnight. After the reaction, a saturated aqueous ammonium chloride solution was added to the mixture. Compounds were extracted with chloroform, and the organic layer was dried with sodium sulfate. The solvent was removed under reduced pressure. Purification by silica gel flash-chromatography (hexane / ethyl acetate=1 / 1) yielded 1 (1.79 g,9.6 mmol, 97%) as a colorless oil. 1H NMR (300 MHz, CDCl3) δ; 4.42(broad s, 1H), 3.91-3.73 (m, 4H), 2.32 (broad s, 1H), 2.24-2.13 (m,1H), 1.96 (broad s, 1H), 1.45 (s, 9H). 13C NMR (75 MHz, CDCl3) δ;157.1, 80.1, 66.4, 63.5, 46.6, 28.2, 17.9. MS (FAB+) m/z; 188([M+H]+). HRMS (FAB+) m/z; 188.1282 (Calcd: 188.1287 forC9H18NO3+). [α]D20= -20.59 (c=1.19, CHCl3, determined on asample with 99.5% e.e.), lit.13 [α]D= -20.3 (c=0.72, CHCl3). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping