| 76% |
With potassium hydroxide; In 5,5-dimethyl-1,3-cyclohexadiene; ethyl acetate; |
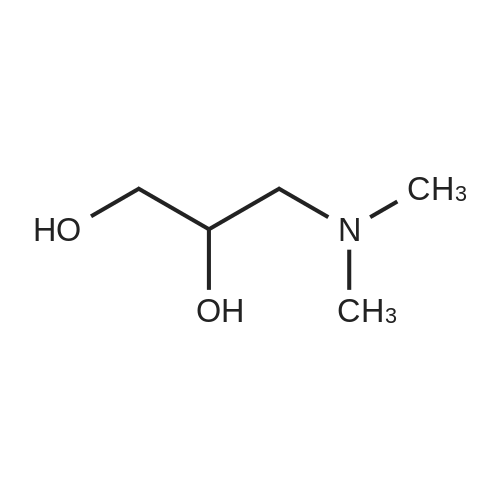
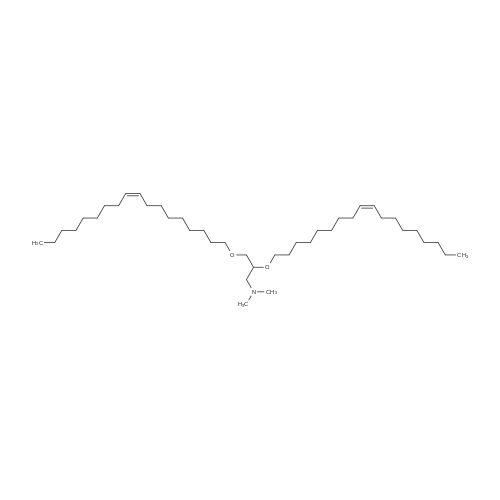
Example 1 2,3 -dioleyloxy-1-(N,N-dimethylamino)propane (1). To a three-necked, 2-liter round bottom flask equipped with a Dean-Stork trap were added 3 -dimethylamino-1,2 -propanediol (6.08 g, 51.1 mmoles), xylene (1300 ml) and KOH (8.0 g). The solution was refluxed for 2 hours while removing water azeotropically via the Dean-Stork trap. Oleyl mesylate (40.0 g, 115.6 mmoles) in 100 ml xylene was added to the reaction mixture drop-wise in 30 minutes. Refluxing was continued for 3 hours and the reaction mixture concentrated to a gum. The gum was triturated with 400 ml hexane and filtered. The solid was washed with 100 ml hexane followed with 200 ml ethyl acetate. The filtrates were combined, concentrated and subjected to f lash chromatography. 2,3-dioleyloxy-1- (N, N-dimethylamino) propane was obtained as a colorless oil in 76percent yield, TLC: Rf =0.37 (Silica gel: 5percent EtOAc: hexane); IR: 2925, 2850, 1469, 1120, 1040 cm-1; H-NMR (CDCl3) delta 5.35 (t, 4H), 4.13 (q, 1H) 3.4-3.65 (m, 6H), 2.35-2.45 (m, 2H), 2.25 (S, 6H), 1.95-2.05 (m, 8H), 1.5-1.65 (m, 4H), 1.2-1.45 (m, 4H) 0.9 (t, 6H). |
| 95 g |
|
[0217] 1,2-Dioleyloxy-3-dimethylaminopropane (DODMA) DLinDMA was synthesized in the same manner, exceptthat oleyl mesylate was replaced with linoley mesylate.[0218] Benzene (800 mL) was added to sodium hydride (52g, 95percent, 2.06 mol) in a 3L pear-shaped round bottom flaskwith a stir bar under argon. A solution of N,N-dimethylaminopropane-1,2-diol (28.1g, 234.8 mmol) in benzene (200mL)was slowly added to the reaction flask under argon, rinsing with a further 50mL of benzene and allowed to stir for 10minutes. [0219] Oleyl mesylate (200.3g, 578.9 mmol) in benzene (200mL) was added to the reaction mixture under argon andrinsed with a further 1200mL of benzene. The reaction mixture was allowed to reflux under argon overnight. [0220] The reaction mixture was transferred to 4L erlenmeyer flask and ethanol (100 mL) was added slowly underargon to quench unreacted sodium hydride. Additional ethanol (1300 mL) was added to give a total ethanol content of1400mL such that benzene:ethanol is 1:1. The reaction mixture (800mL) was aliquoted to a 2L separatory funnel and240mL water was added (benzene:ethanol:water 1:1:0.6 v/v). The organic phase was collected and the aqueous layerwas re-extracted with benzene (100mL).[0221] Oleyl mesylate (200.3g, 578.9 mmol) in benzene (200mL) was added to the reaction mixture under argon andrinsed with a further 1200mL of benzene. The reaction mixture was allowed to reflux under argon overnight. This stepwas repeated again. [0222] The combined organic fractions were dried with anhydrous magnesium sulphate (30g) and filtered under vacuumusing a sintered glass funnel. Solvent was removed on a rotovap (water bath 50 - 60hC). The viscous oily product wasredissolved in dichloromethane (300 mL) and vacuum filtered through a sintered glass funnel with a filter paper andsilica gel 60 (80g, 230 - 400 mesh). Dichloromethane was removed on a rotovap at 50 - 60hC. [0223] The product was purified by column chromatography. A total of 151 g product was divided into two ?75g aliquotsand loaded onto two 600g silica gel 60 columns. The product was dissolved in 2percent MeOH in dichloromethane (?1:1 w/v)prior to loading onto the column. 2percent MeOH in dichloromethane (?1 L) was used until product came out. Approximately1 L of 5percent, 7.5percent and then 10percent MeOH in dichloromethane were used to elute the columns collecting ?200 mL fractions.[0224] Fractions with a top or bottom spot on TLC (impurity) and product were rotovaped separately from the purefractions. Impure DODMA was collected from other batches, added together, and put down a column a second time topurify. The yield of DODMA was 95g. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping